Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-09-28 , DOI: 10.1016/j.jhazmat.2020.124035 Qiang Zeng , Liang Hu , Hui Zhong , Zhiguo He , Wei Sun , Daoling Xiong
|
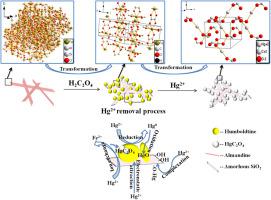
Efficient removal of Hg2+ from aqueous solution is key for environmental protection and human health. Herein, a novel composite of nano humboldtine decorated almandine was synthesized from almandine for the removal of Hg2+. Results showed that the Hg2+ removal process followed pseudo-second-order kinetic model and Langmuir equation, and the maximum adsorption capacity was 575.17 mg/g. Furthermore, Hg2+ removal by the composite was pH-dependent and low pH value facilitated the removal of Hg2+. SEM and HADDF-STEM results suggested a new rod morphology was generated and the adsorbed mercury was mainly enriched into this structure after reaction with Hg2+ solution. The removal mechanisms of Hg2+ by the composite was pH dependent, and included ion exchange, surface complexation, reduction and oxidation. Our results demonstrated that the composite was an ideal material for Hg2+ removal and the transformation ways of mercury related species could be a significant but currently underestimated pathway in natural and engineered systems.
中文翻译:

新型纳米金刚烷修饰的金刚烷胺(NHDA)的新型复合材料可有效去除水溶液中的Hg 2+:离子交换,还原氧化和吸附
从水溶液中有效去除Hg 2+是环境保护和人类健康的关键。在此,从铝金刚烷中合成了一种新型的由纳米金刚烷修饰的铝金刚烷复合物,用于去除Hg 2+。结果表明,Hg 2+的去除过程遵循拟二级动力学模型和Langmuir方程,最大吸附量为575.17 mg / g。此外,复合材料对Hg 2+的去除取决于pH,低pH值有助于去除Hg 2+。SEM和HADDF-STEM结果表明,与Hg 2+反应后,生成了新的棒状形态,并且吸附的汞主要富集到该结构中解。复合材料对Hg 2+的去除机理与pH有关,包括离子交换,表面络合,还原和氧化。我们的结果表明,该复合材料是去除Hg 2+的理想材料,并且与汞有关的物种的转化方式在自然和工程系统中可能是重要但目前仍被低估的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号