European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-09-28 , DOI: 10.1016/j.ejmech.2020.112874 Min Wang 1 , Tongtong Liu 1 , Shiming Chen 1 , Mingfei Wu 1 , Jianfei Han 1 , Zeng Li 1
|
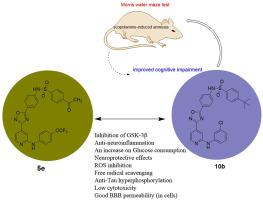
Pleiotropic intervention has prominent advantages for complex pathomechanisms, such as Alzheimer’s disease (AD). In this study, a series of novel 3-(4-pyridyl)-5-(4- sulfamido-phenyl)-1,2,4-oxadiazole derivatives were designed and synthesized following the multitarget-directed ligand-based strategy. All compounds were evaluated for glycogen synthase kinase 3β (GSK-3β) inhibition and antineuroinflammatory and neuroprotective activities. Given that abnormal glucose metabolism plays an important role in AD occurrence and development, the effects of all compounds on glucose consumption in HepG2 cells was evaluated. Compounds 5e and 10b showed good dual potency in GSK-3β inhibition (IC50: 5e = 1.52 μM, 10b = 0.19 μM) and antineuroinflammatory potency (IC50: 5e = 0.47 ± 0.64 μM, 10b = 6.94 ± 2.33 μM). The effect of compound 10b on glucose consumption was higher than that of positive drug metformin. These compounds exerted a certain neuroprotective effect. Compound 10b dramatically reduced Aβ-induced Tau hyperphosphorylation, thus inhibiting GSK-3β at the cellular level. Notably, compounds 5e and 10b exhibited good inhibitory effects on the formation of intracellular reactive oxygen species (ROS). Moreover, these compounds displayed proper blood–brain barrier permeability and lacked neurotoxicity up to 50 μM concentration. Finally, in vivo experiments revealed that compound 10b improved cognitive impairment in scopolamine-induced mouse models. Results indicated that compound 10b deserves further study as a multifunctional lead compound.
中文翻译:

新型GSK-3β抑制剂3-(4-吡啶基)-5-(4-磺酰胺基苯基)-1,2,4-恶二唑衍生物的设计和合成及其作为多功能抗阿尔茨海默病药物的潜力
多效性干预对于复杂的病理机制具有显着的优势,例如阿尔茨海默病(AD)。在本研究中,按照多靶点定向配体策略设计并合成了一系列新型3-(4-吡啶基)-5-(4-磺酰胺基苯基)-1,2,4-恶二唑衍生物。对所有化合物的糖原合成酶激酶 3β (GSK-3β) 抑制以及抗神经炎症和神经保护活性进行了评估。鉴于异常的葡萄糖代谢在AD的发生和发展中起着重要作用,评估了所有化合物对HepG2细胞葡萄糖消耗的影响。化合物5e和10b在GSK-3β抑制(IC 50 : 5e = 1.52 μM, 10b = 0.19 μM)和抗神经炎症效力(IC 50 : 5e = 0.47 ± 0.64 μM, 10b = 6.94 ± 2.33 μM)方面表现出良好的双重效力。化合物10b对葡萄糖消耗的影响高于阳性药物二甲双胍。这些化合物发挥了一定的神经保护作用。化合物10b显着降低 Aβ 诱导的 Tau 过度磷酸化,从而在细胞水平抑制 GSK-3β。值得注意的是,化合物5e和10b对细胞内活性氧(ROS)的形成表现出良好的抑制作用。此外,这些化合物表现出适当的血脑屏障渗透性,并且在浓度高达 50 μM 时没有神经毒性。最后,体内实验表明,化合物10b可以改善东莨菪碱诱导的小鼠模型的认知障碍。 结果表明,化合物10b作为多功能先导化合物值得进一步研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号