Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2020-09-25 , DOI: 10.1016/j.jclepro.2020.124374 Tao Luo , Hao Wang , Long Chen , Jinjun Li , Feng Wu , Danna Zhou
|
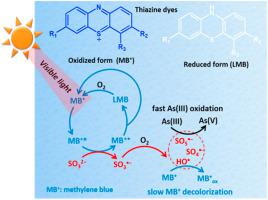
Recently, the advanced oxidation processes (AOPs) based on sulfate radical (SO4•⁻) using sulfite has received increasing concerns. Metal-free activation of sulfite is still in urgent demands from the perspective of cleaner production since the reported efficient systems containing sulfite has been relied on the transition metals. In this work, methylene blue (MB⁺), a well-known photosensitizer and thiazine dye contaminant, was utilized to activate sulfite for the efficient photooxidation of arsenite (As(III)) to arsenate (As(V)) under visible light (LED lamps with wavelength 640 nm) and sunlight, respectively. The initial oxidation rates of 5–40 μM As(III) are 28-fold–116-fold as that of the decolorization of 0.27 μM MB⁺ with 50 μM sulfite at pH 7.3. The maximum stoichiometric ratio MB⁺: As(III): sulfite was obtained to be 1 : 142: 247, suggesting an efficient utilization of both MB⁺ and sulfite as waste resources. The electrical energy per order (EE/O) index was calculated to be 1.83 × 10−3 kWh L−1 for the visible light-MB+-sulfite system, which is much less than those reported systems. Oxysulfur radicals (mainly peroxymonosulfate radical, SO5•⁻) produced in this photochemical system are responsible for As(III) oxidation. The other two common thiazine dyes (thionine and Azure B) are also capable of activating sulfite for As(III) oxidation due to the same chromophores. Thus the novel strategy of using waste sulfite and the thiazine dyes is promising for As(III) oxidation under sunlight, with which low-concentration wastes of thiazine dye and sulfite can be utilized to achieve the goal of cleaner production.
中文翻译:

可见光驱动的亚砷酸盐,亚硫酸盐和噻嗪染料的氧化:利用废物处理废物的新策略
最近,基于硫酸根(SO 4 •⁻)使用亚硫酸盐已引起越来越多的关注。从更清洁的生产的角度来看,亚硫酸盐的无金属活化仍然是紧迫的需求,因为已报道的含有亚硫酸盐的有效体系已经依赖于过渡金属。在这项工作中,使用亚甲基蓝(MBizer)(一种众所周知的光敏剂和噻嗪染料污染物)来活化亚硫酸盐,以便在可见光下有效地将亚砷酸盐(As(III))光氧化为砷酸盐(As(V))(波长分别为640 nm和日光的LED灯。5-40μMAs(III)的初始氧化速率是0.27μMMB⁺与50μM亚硫酸盐(pH 7.3)脱色的初始氧化速率的28倍至116倍。得到的最大化学计量比MB 3 ∶As(Ⅲ):亚硫酸盐为1∶142∶247,这表明MB 3和亚硫酸盐作为废物资源均得到有效利用。-MB +-亚硫酸盐可见光系统的功率为-3 kWh L -1,远低于报告的系统。在该光化学系统中产生的氧硫自由基(主要是过氧一硫酸盐自由基,SO 5 • are)是As(III)氧化的原因。由于相同的生色团,其他两种常见的噻嗪染料(硫氨酸和天青B)也能够活化亚硫酸盐进行As(III)氧化。因此,利用废亚硫酸盐和噻嗪染料的新策略有望在阳光下氧化As(III),利用该低浓度的噻嗪染料和亚硫酸盐废物达到清洁生产的目的。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号