当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New peptide architectures through C-H activation stapling between tryptophan-phenylalanine/tyrosine residues.
Nature Communications ( IF 14.7 ) Pub Date : 2015-May-21 , DOI: 10.1038/ncomms8160 Lorena Mendive-Tapia , Sara Preciado , Jesús García , Rosario Ramón , Nicola Kielland , Fernando Albericio , Rodolfo Lavilla
Nature Communications ( IF 14.7 ) Pub Date : 2015-May-21 , DOI: 10.1038/ncomms8160 Lorena Mendive-Tapia , Sara Preciado , Jesús García , Rosario Ramón , Nicola Kielland , Fernando Albericio , Rodolfo Lavilla
|
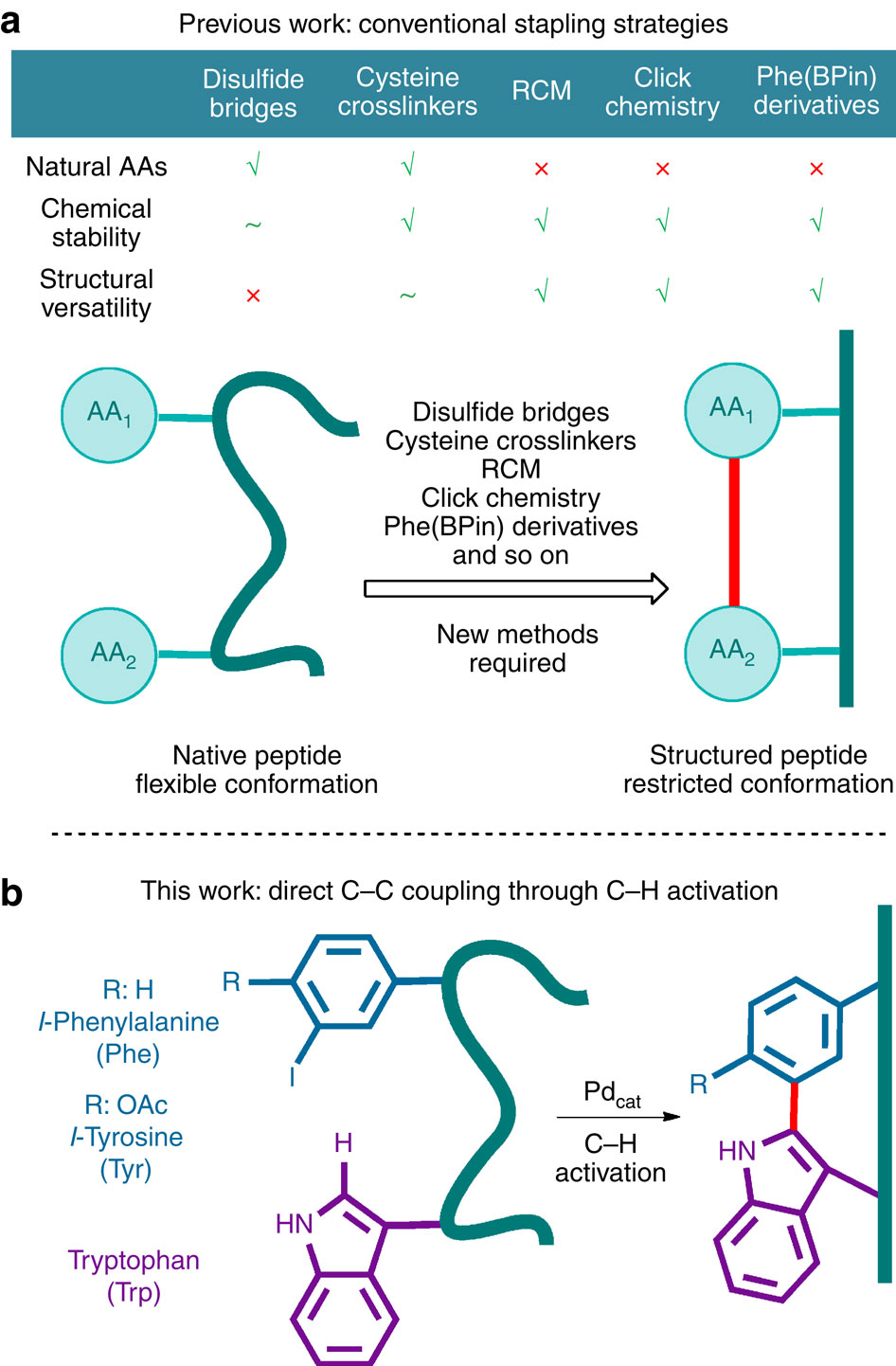
Natural peptides show high degrees of specificity in their biological action. However, their therapeutical profile is severely limited by their conformational freedom and metabolic instability. Stapled peptides constitute a solution to these problems and access to these structures lies on a limited number of reactions involving the use of non-natural amino acids. Here, we describe a synthetic strategy for the preparation of unique constrained peptides featuring a covalent bond between tryptophan and phenylalanine or tyrosine residues. The preparation of such peptides is achieved in solution and on solid phase directly from the corresponding sequences having an iodo-aryl amino acid through an intramolecular palladium-catalysed C-H activation process. Moreover, complex topologies arise from the internal stapling of cyclopeptides and double intramolecular arylations within a linear peptide. Finally, as a proof of principle, we report the application to this new stapling method to relevant biologically active compounds.
中文翻译:

通过色氨酸-苯丙氨酸/酪氨酸残基之间的CH活化吻合的新肽结构。
天然肽在其生物作用中显示出高度的特异性。但是,它们的治疗方式受到其构象自由度和代谢不稳定的严重限制。装订的肽构成了解决这些问题的方法,进入这些结构的方法取决于有限数量的涉及使用非天然氨基酸的反应。在这里,我们描述了一种制备独特约束肽的合成策略,该肽具有色氨酸和苯丙氨酸或酪氨酸残基之间的共价键。通过在分子内钯催化的CH活化过程中,在溶液中和在固相上直接从具有碘-芳基氨基酸的相应序列中制备此类肽。而且,复杂的拓扑结构是由环肽的内部装订和线性肽内的双重分子内芳基化引起的。最后,作为原理上的证明,我们报告了这种新的装订方法对相关生物活性化合物的应用。
更新日期:2015-05-25
中文翻译:

通过色氨酸-苯丙氨酸/酪氨酸残基之间的CH活化吻合的新肽结构。
天然肽在其生物作用中显示出高度的特异性。但是,它们的治疗方式受到其构象自由度和代谢不稳定的严重限制。装订的肽构成了解决这些问题的方法,进入这些结构的方法取决于有限数量的涉及使用非天然氨基酸的反应。在这里,我们描述了一种制备独特约束肽的合成策略,该肽具有色氨酸和苯丙氨酸或酪氨酸残基之间的共价键。通过在分子内钯催化的CH活化过程中,在溶液中和在固相上直接从具有碘-芳基氨基酸的相应序列中制备此类肽。而且,复杂的拓扑结构是由环肽的内部装订和线性肽内的双重分子内芳基化引起的。最后,作为原理上的证明,我们报告了这种新的装订方法对相关生物活性化合物的应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号