当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Specific Host-Guest Interactions in the Crown Ether Complexes with K+ and NH4+ Revealed from the Vibrational Relaxation Dynamics of the Counter-anion.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-09-23 , DOI: 10.1021/acs.jpcb.0c07032 Dexia Zhou 1 , Hongxing Hao 1 , Yinhua Ma 1 , Hongmei Zhong 2 , Ya'nan Dai 2 , Kaicong Cai 2 , Somnath Mukherjee 1 , Jing Liu 1 , Hongtao Bian 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-09-23 , DOI: 10.1021/acs.jpcb.0c07032 Dexia Zhou 1 , Hongxing Hao 1 , Yinhua Ma 1 , Hongmei Zhong 2 , Ya'nan Dai 2 , Kaicong Cai 2 , Somnath Mukherjee 1 , Jing Liu 1 , Hongtao Bian 1
Affiliation
|
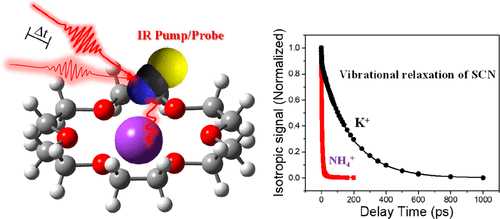
The specific host–guest interactions in the corresponding complexes of K+ and NH4+ with typical crown ethers were investigated by using FTIR and ultrafast IR spectroscopies. The counteranions, i.e., SCN–, were employed as a local vibrational probe to report the structural dynamics of the complexation. It was found that the vibrational relaxation dynamics of the SCN– was strongly affected by the cations confined in the cavities of the crown ethers. The time constant of the vibrational population decay of SCN– in the complex of NH4+ with the 18-crown-6 was determined to be 6 ± 2 ps, which is ∼30 times faster than that in the complex of K+ with the crown ethers. Control experiments showed that the vibrational population decay of SCN– depended on the size of the cavities of the crown ethers. A theoretical calculation further indicated that the nitrogen atom of SCN– showed preferential coordination to the K+ ions hosted by the crown ethers, while the NH4+ can form hydrogen bonds with the oxygen atoms in the studied crown ethers. The geometric constraints formed in the complex of crown ethers can cause a specific interaction between the NH4+ and SCN–, which can facilitate the intermolecular vibrational energy redistribution of the SCN–.
中文翻译:

从抗衡阴离子的振动弛豫动力学揭示了冠醚与K +和NH4 +配合物中特定的宿主-客体相互作用。
使用FTIR和超快红外光谱研究了K +和NH 4 +与典型冠醚的相应配合物中的特定客体-客体相互作用。对阴离子,即,SCN - ,被聘为当地振动探头报告络合的结构动力学。研究发现,SCN –的振动弛豫动力学受到冠醚腔中所含阳离子的强烈影响。NH 4 +与18冠6配合物中SCN –的振动种群衰减的时间常数确定为6±2 ps,比K +配合物快约30倍。与冠醚。对照实验表明,SCN的振动粒子数-依赖于冠醚腔的大小。理论计算进一步指出,SCN的氮原子-表明优先协调到第K +由冠醚托管离子,而NH 4 +可以与在所研究的冠醚的氧原子形成氢键。形成在复合冠醚的几何约束可能导致特定的相互作用NH之间4 +和SCN - ,这可以有助于所述SCN的分子间的振动能量再分配- 。
更新日期:2020-10-16
中文翻译:

从抗衡阴离子的振动弛豫动力学揭示了冠醚与K +和NH4 +配合物中特定的宿主-客体相互作用。
使用FTIR和超快红外光谱研究了K +和NH 4 +与典型冠醚的相应配合物中的特定客体-客体相互作用。对阴离子,即,SCN - ,被聘为当地振动探头报告络合的结构动力学。研究发现,SCN –的振动弛豫动力学受到冠醚腔中所含阳离子的强烈影响。NH 4 +与18冠6配合物中SCN –的振动种群衰减的时间常数确定为6±2 ps,比K +配合物快约30倍。与冠醚。对照实验表明,SCN的振动粒子数-依赖于冠醚腔的大小。理论计算进一步指出,SCN的氮原子-表明优先协调到第K +由冠醚托管离子,而NH 4 +可以与在所研究的冠醚的氧原子形成氢键。形成在复合冠醚的几何约束可能导致特定的相互作用NH之间4 +和SCN - ,这可以有助于所述SCN的分子间的振动能量再分配- 。













































 京公网安备 11010802027423号
京公网安备 11010802027423号