Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-09-23 , DOI: 10.1007/s10593-020-02776-4 Lyudmila M. Potikha , Volodymyr S. Brovarets
|
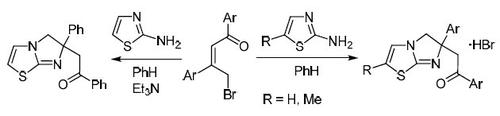
We propose a new method for assembling the imidazo[2,1-b][1,3]thiazole system, based on the reaction of (2Z)-1,3-diaryl-4-bromobut-2-en-1-one derivatives with 2-aminothiazoles. The outcome of this reaction depends on the structure of the starting bromo ketone: when electron-withdrawing substituents are present in the structure of the ketone, a competing reaction occurs, which leads to the formation of 2,5-diarylfurans. Screening for antitumor activity has been performed in the case of 1-phenyl-2-(6-phenyl-5,6-dihydroimidazo[2,1-b]- thiazol-6-yl)ethanone and this compound has shown moderate ability to suppress the growth of kidney cancer cells, with a weaker effect on the cell lines of prostate cancer, colon cancer, and leukemia.
中文翻译:

咪唑并[2,1-b] [1,3]噻唑的合成-潜在的抗癌药,来自γ-溴二甲酮
基于(2 Z)-1,3-二芳基-4-溴丁-2-烯-1-的反应,我们提出了一种新的咪唑并[2,1- b ] [1,3]噻唑体系的组装方法一种具有2-氨基噻唑的衍生物。该反应的结果取决于起始溴代酮的结构:当吸电子的取代基存在于酮的结构中时,发生竞争反应,这导致形成2,5-二芳基呋喃。在1-苯基-2-(6-苯基-5,6-二氢咪唑并[2,1- b ]-噻唑-6-基)乙酮的情况下,已经进行了抗肿瘤活性的筛选,并且该化合物显示出适度的抗肿瘤活性。抑制肾癌细胞的生长,对前列腺癌,结肠癌和白血病的细胞系作用较弱。






























 京公网安备 11010802027423号
京公网安备 11010802027423号