Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-09-22 , DOI: 10.1016/j.apcatb.2020.119569 Wei Liu , Yusen Yang , Lifang Chen , Enze Xu , Jiaming Xu , Song Hong , Xin Zhang , Min Wei
|
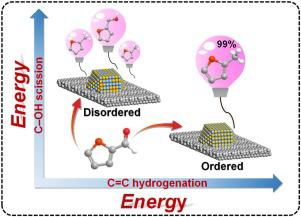
Activation of oxygen-containing functional groups plays a key role in sustainable biomass upgrading and conversion. In this work, a NiMo intermetallic compound (IMC) catalyst was prepared based on layered double hydroxides (LDHs) precursors, which displayed prominent catalytic performance for furfural hydrodeoxygenation (HDO) to 2-methylfuran (2-MF) (yield: 99%) at a rather low hydrogen pressure (0.1 MPa), significantly superior to NiMo alloy, monometallic Ni and other Ni-based catalysts ever reported. CO-IR, STEM, EXAFS and XANES give direct evidences that the atomically-ordered Ni/Mo sites in NiMo IMC determine the uniform bridging-type adsorption mode of C = O bond in furfural whilst adsorption of furan ring is extremely suppressed. In situ FT-IR and DFT calculation further substantiate that ordered Ni-Mo bimetallic sites of IMC, in contrast to the random atomic sequence in NiMo alloy, facilitate the activation and cleavage of C―OH bond in the intermediate (furfuryl alcohol, FOL), accounting for the production of 2-MF. This work demonstrates the decisive effect of atomically-ordered active sites in IMC catalyst on activation of oxygen-containing functional groups and product selectivity, which can be extended to catalytic upgrading of biomass-derived platform molecules.
中文翻译:

NiMo金属间化合物中对糠醛低压加氢脱氧的原子排序活性位点
含氧官能团的活化在可持续的生物质升级和转化中起关键作用。在这项工作中,基于层状双氢氧化物(LDHs)前驱体制备了NiMo金属间化合物(IMC)催化剂,该催化剂对糠醛加氢脱氧(HDO)生成2-甲基呋喃(2-MF)具有突出的催化性能(收率:99%)在相当低的氢气压力(0.1 MPa)下,据报道优于NiMo合金,单金属Ni和其他基于Ni的催化剂。CO-IR,STEM,EXAFS和XANES提供了直接的证据,表明NiMo IMC中原子序排列的Ni / Mo位点决定了糠醛中C = O键的均匀桥接型吸附模式,而呋喃环的吸附却受到了极大的抑制。原位FT-IR和DFT计算进一步证实了IMC的有序Ni-Mo双金属位点,与NiMo合金中的随机原子序列相反,促进了中间体(糠醇,FOL)中C-OH键的活化和裂解,用于生产2-MF。这项工作证明了IMC催化剂中原子序的活性位对含氧官能团的活化和产物选择性的决定性作用,该作用可以扩展到生物质衍生平台分子的催化提质。






























 京公网安备 11010802027423号
京公网安备 11010802027423号