当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiospecific generation and trapping reactions of aryne atropisomers
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-21 , DOI: 10.1021/jacs.0c08218 Yun-Long Wei 1 , Guillaume Dauvergne 1 , Jean Rodriguez 1 , Yoann Coquerel 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-21 , DOI: 10.1021/jacs.0c08218 Yun-Long Wei 1 , Guillaume Dauvergne 1 , Jean Rodriguez 1 , Yoann Coquerel 1
Affiliation
|
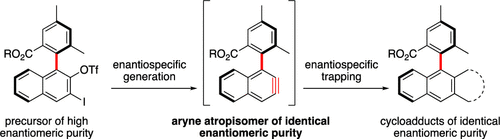
Enantioenriched aryne atropisomers having a biaryl stereogenic axis vicinal to the reactive triple bond are demonstrated to exist. These reaction intermediates are easily produced in situ and can undergo the standard aryne cycloaddition chemistry in an enantiospecific manner. Notably, the aryne atropisomers herein have allowed the practical syntheses of a small nanographene, and some triptycene and anthracene derivatives that embed stereogenic axes of controlled absolute configurations.
中文翻译:

芳炔阻转异构体的对映特异性生成和捕获反应
已证明存在具有邻近反应性三键的联芳基立体轴的对映体富集的芳炔阻转异构体。这些反应中间体很容易就地生产,并且可以以对映特异性方式进行标准的芳炔环加成化学反应。值得注意的是,本文中的芳炔阻转异构体允许实际合成小纳米石墨烯,以及一些嵌入受控绝对构型的立体轴的三苯乙烯和蒽衍生物。
更新日期:2020-09-21
中文翻译:

芳炔阻转异构体的对映特异性生成和捕获反应
已证明存在具有邻近反应性三键的联芳基立体轴的对映体富集的芳炔阻转异构体。这些反应中间体很容易就地生产,并且可以以对映特异性方式进行标准的芳炔环加成化学反应。值得注意的是,本文中的芳炔阻转异构体允许实际合成小纳米石墨烯,以及一些嵌入受控绝对构型的立体轴的三苯乙烯和蒽衍生物。































 京公网安备 11010802027423号
京公网安备 11010802027423号