Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2020-09-21 , DOI: 10.1016/j.abb.2020.108590 Poonam Dhankhar , Vikram Dalal , Jai Krishna Mahto , Bhola Ram Gurjar , Shailly Tomar , Ashwani Kumar Sharma , Pravindra Kumar
|
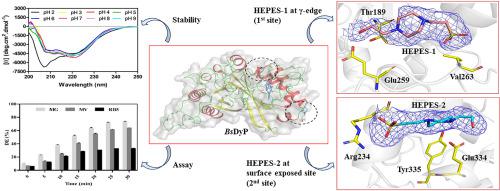
The dye-decolorizing peroxidases (DyPs) belong to a unique heme peroxidase family for their biotechnological potential to detoxify synthetic dyes. In this work, we have biochemically and structurally characterized the dye-decolorizing peroxidase from Bacillus subtilis (BsDyP). The biochemical studies of BsDyP demonstrate that pH 4.0 is optimum for the oxidation of malachite green (MG) and methyl violet (MV). However, it oxidizes the MG with higher catalytic efficiency (kcat/Km = 6.3 × 102 M−1s−1), than MV (kcat/Km = 5.0 × 102 M−1s−1). While reactive black 5 (RB5) is oxidized at pH 3.0 with the catalytic efficiency of kcat/Km = 3.6 × 102 M−1s−1. The calculated thermodynamic parameters by isothermal titration calorimetry (ITC) reveal the feasibility and spontaneity of dyes binding with BsDyP. Further, the crystal structures of a HEPES bound and unbound of BsDyP provide insight into the probable binding sites of the substrates. In BsDyP-HEPES bound structure, the HEPES-1 molecule is found in the heme cavity at the γ-edge, and another HEPES-2 molecule is bound ~16 Å away from the heme that is fenced by Ile231, Arg234, Ser235, Asp239, Glu334, and surface-exposed Tyr335 residues. Furthermore, the molecular docking, simulation, and MMPBSA studies support the binding of dyes at both the sites of BsDyP and produce lower-energy stable BsDyP-dyes complexes. Here, the BsDyP study allows the identification of its two potential binding sites and shows the oxidation of a variety of dyes. Structural and functional insight of BsDyP will facilitate its engineering for the improved decolorization of dyes.
中文翻译:

枯草芽孢杆菌的染料脱色过氧化物酶的表征
染料脱色过氧化物酶(DyPs)属于独特的血红素过氧化物酶家族,因其具有生物化学潜力,可以对合成染料进行解毒。在这项工作中,我们已经对枯草芽孢杆菌(Bs DyP)的染料脱色过氧化物酶进行了生化和结构表征。Bs DyP的生化研究表明,pH 4.0最适合用于孔雀石绿(MG)和甲基紫(MV)的氧化。但是,与MV(k cat / K m = 5.0×10 2 M相比),它以更高的催化效率(k cat / K m = 6.3×10 2 M -1 s -1)氧化MG。-1 s -1)。反应性黑5(RB5)在pH 3.0时被氧化,催化效率为k cat / K m = 3.6×10 2 M -1 s -1。通过等温滴定热法(ITC)计算的热力学参数揭示了染料与Bs DyP结合的可行性和自发性。此外,结合和未结合Bs DyP的HEPES的晶体结构提供了对底物可能的结合位点的了解。在BSDyP-HEPES结合的结构,HEPES-1分子在γ边缘的血红素腔中发现,另一个HEPES-2分子与被Ile231,Arg234,Ser235,Asp239包围的血红素结合约16Å。 Glu334和表面暴露的Tyr335残基。此外,分子对接,模拟和MMPBSA研究支持染料在Bs DyP的两个位点结合,并产生能量稳定的Bs DyP-染料复合物。在这里,Bs DyP研究允许鉴定其两个潜在的结合位点,并显示了各种染料的氧化。Bs DyP的结构和功能洞察力将有助于其工程学,以改善染料的脱色效果。































 京公网安备 11010802027423号
京公网安备 11010802027423号