当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pictet–Spengler reaction based on in situ generated α-amino iminium ions through the Heyns rearrangement
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-09-18 , DOI: 10.1039/d0qo00722f Jun-xiu Liang 1, 2, 3, 4, 5 , Guo-bin Yang 1, 2, 3, 4 , Yong-po Zhang 1, 2, 3, 4 , Dong-dong Guo 1, 2, 3, 4 , Jin-zhong Zhao 1, 2, 3, 4 , Guang-xun Li 4, 5, 6, 7, 8 , Zhuo Tang 4, 5, 6, 7, 8
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-09-18 , DOI: 10.1039/d0qo00722f Jun-xiu Liang 1, 2, 3, 4, 5 , Guo-bin Yang 1, 2, 3, 4 , Yong-po Zhang 1, 2, 3, 4 , Dong-dong Guo 1, 2, 3, 4 , Jin-zhong Zhao 1, 2, 3, 4 , Guang-xun Li 4, 5, 6, 7, 8 , Zhuo Tang 4, 5, 6, 7, 8
Affiliation
|
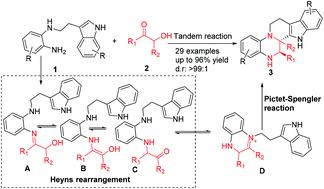
A useful tandem reaction via the Heyns rearrangement and Pictet–Spengler reaction was developed which ensured the synthesis of complex N-heteropolycycles containing tetrahydro-β-carboline with high yield (up to 96%) and dr (99 : 1). The reaction proceeded smoothly with a catalytic Brønsted acid as a catalyst. The reaction mechanism was investigated which demonstrated that the tandem reaction proceeded due to the in situ produced α-amino iminium ions via the Heyns rearrangement.
中文翻译:

通过Heyns重排基于原位产生的α-氨基亚胺离子的Pictet-Spengler反应
通过Heyns重排和Pictet-Spengler反应开发了有用的串联反应,该反应可确保合成高产率(最高96%)和dr(99:1)的含有四氢-β-咔啉的复杂N-杂多环。用催化布朗斯台德酸作为催化剂使反应顺利进行。研究了反应机理,结果表明串联反应是由于通过Heyns重排原位产生的α-氨基亚胺离子而进行的。
更新日期:2020-10-13
中文翻译:

通过Heyns重排基于原位产生的α-氨基亚胺离子的Pictet-Spengler反应
通过Heyns重排和Pictet-Spengler反应开发了有用的串联反应,该反应可确保合成高产率(最高96%)和dr(99:1)的含有四氢-β-咔啉的复杂N-杂多环。用催化布朗斯台德酸作为催化剂使反应顺利进行。研究了反应机理,结果表明串联反应是由于通过Heyns重排原位产生的α-氨基亚胺离子而进行的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号