当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stepwise Recovery of Valuable Metals from Spent Lithium Ion Batteries by Controllable Reduction and Selective Leaching and Precipitation
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-09-17 , DOI: 10.1021/acssuschemeng.0c04106 Yingchao Zhang 1 , Wenqiang Wang 1 , Jiehui Hu 1 , Tao Zhang 1, 2 , Shengming Xu 1, 3, 4
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-09-17 , DOI: 10.1021/acssuschemeng.0c04106 Yingchao Zhang 1 , Wenqiang Wang 1 , Jiehui Hu 1 , Tao Zhang 1, 2 , Shengming Xu 1, 3, 4
Affiliation
|
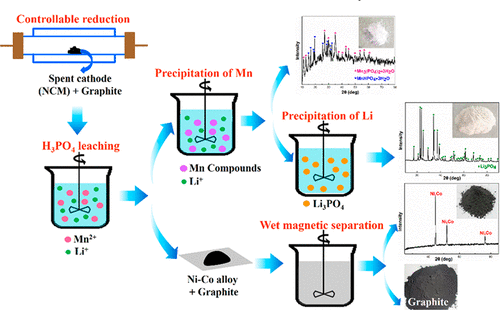
The remarkably increasing consumption of lithium ion batteries (LIBs) has been an issue worldwide for the contained valuable metals and intrinsic toxicity in spent LIBs. Here, a novel process including selective leaching and precipitation has been developed for recovery of spent LIBs. The carbothermic reduction of spent LiNixCoyMn1–x–yO2 was applied to control the reduction degree of target metallic elements. Afterward, H3PO4 solution was utilized to selectively leach 99.1% of Li and 96.3% of Mn, and excellent leaching selectivity was achieved for that merely 4.5% of Co and 1.2% of Ni were dissolved. After increasing the concentrations of Li and Mn to 23.14 and 53.03 g/L by two-stage cross-current leaching, Li and Mn were precipitated and recovered in sequence by simply adjusting pH of the leaching lixivium, and compounds of Mn3(PO4)2·3H2O and MnHPO4·3H2O as well as Li3PO4 were obtained. The Ni–Co alloy contained in the filtration residue could be separated by wet magnetic separation and further recovered. It was the first time to synchronously leach Li and Mn while Co and Ni were separated simultaneously in a single leaching operation, which could greatly benefit the subsequent separation and recovery. The selective leaching and precipitation are efficient and facile and enjoy superiority over conventional processes of recycling spent LIBs.
中文翻译:

通过可控制的还原,选择性浸出和沉淀法逐步从废锂离子电池中回收有价金属
锂离子电池(LIB)的消耗量显着增加已成为世界范围内的问题,原因是废旧LIB中所含的贵重金属和内在毒性。在这里,已经开发出一种包括选择性浸出和沉淀的新方法来回收用过的LIB。对废LiNi x Co y Mn 1– x – y O 2进行碳热还原可控制目标金属元素的还原度。之后,H 3 PO 4利用该溶液选择性地浸出99.1%的Li和96.3%的Mn,由于仅溶解了4.5%的Co和1.2%的Ni而获得了极好的浸出选择性。通过两阶段错流浸提将Li和Mn的浓度分别提高至23.14和53.03 g / L之后,只需调节浸出的pH值和Mn 3(PO 4)2 ·3H 2 O和MnHPO 4 ·3H 2 O以及Li 3 PO 4获得了。过滤残留物中所含的Ni-Co合金可通过湿式磁选法分离并进一步回收。这是第一次同步浸出Li和Mn,同时一次浸出同时分离Co和Ni的方法,这将大大有利于后续的分离和回收。选择性浸出和沉淀是有效且容易的,并且比回收用过的LIB的常规方法优越。
更新日期:2020-10-21
中文翻译:

通过可控制的还原,选择性浸出和沉淀法逐步从废锂离子电池中回收有价金属
锂离子电池(LIB)的消耗量显着增加已成为世界范围内的问题,原因是废旧LIB中所含的贵重金属和内在毒性。在这里,已经开发出一种包括选择性浸出和沉淀的新方法来回收用过的LIB。对废LiNi x Co y Mn 1– x – y O 2进行碳热还原可控制目标金属元素的还原度。之后,H 3 PO 4利用该溶液选择性地浸出99.1%的Li和96.3%的Mn,由于仅溶解了4.5%的Co和1.2%的Ni而获得了极好的浸出选择性。通过两阶段错流浸提将Li和Mn的浓度分别提高至23.14和53.03 g / L之后,只需调节浸出的pH值和Mn 3(PO 4)2 ·3H 2 O和MnHPO 4 ·3H 2 O以及Li 3 PO 4获得了。过滤残留物中所含的Ni-Co合金可通过湿式磁选法分离并进一步回收。这是第一次同步浸出Li和Mn,同时一次浸出同时分离Co和Ni的方法,这将大大有利于后续的分离和回收。选择性浸出和沉淀是有效且容易的,并且比回收用过的LIB的常规方法优越。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号