Cell Metabolism ( IF 27.7 ) Pub Date : 2020-09-18 , DOI: 10.1016/j.cmet.2020.09.006 Satotaka Omori 1 , Teh-Wei Wang 1 , Yoshikazu Johmura 1 , Tomomi Kanai 1 , Yasuhiro Nakano 2 , Taketomo Kido 2 , Etsuo A Susaki 3 , Takuya Nakajima 4 , Shigeyuki Shichino 4 , Satoshi Ueha 4 , Manabu Ozawa 5 , Kisho Yokote 1 , Soichiro Kumamoto 1 , Atsuya Nishiyama 1 , Takeharu Sakamoto 6 , Kiyoshi Yamaguchi 7 , Seira Hatakeyama 7 , Eigo Shimizu 8 , Kotoe Katayama 8 , Yasuhiro Yamada 9 , Satoshi Yamazaki 10 , Kanako Iwasaki 11 , Chika Miyoshi 11 , Hiromasa Funato 12 , Masashi Yanagisawa 13 , Hiroo Ueno 14 , Seiya Imoto 8 , Yoichi Furukawa 7 , Nobuaki Yoshida 15 , Kouji Matsushima 4 , Hiroki R Ueda 3 , Atsushi Miyajima 2 , Makoto Nakanishi 1
|
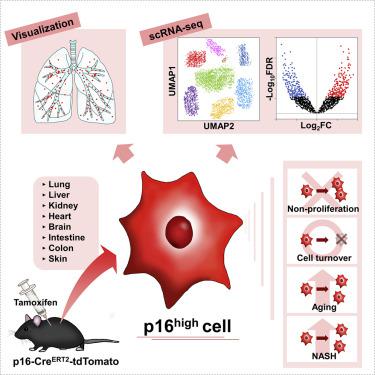
Cell senescence plays a key role in age-associated organ dysfunction, but the in vivo pathogenesis is largely unclear. Here, we generated a p16-CreERT2-tdTomato mouse model to analyze the in vivo characteristics of p16high cells at a single-cell level. We found tdTomato-positive p16high cells detectable in all organs, which were enriched with age. We also found that these cells failed to proliferate and had half-lives ranging from 2.6 to 4.2 months, depending on the tissue examined. Single-cell transcriptomics in the liver and kidneys revealed that p16high cells were present in various cell types, though most dominant in hepatic endothelium and in renal proximal and distal tubule epithelia, and that these cells exhibited heterogeneous senescence-associated phenotypes. Further, elimination of p16high cells ameliorated nonalcoholic steatohepatitis-related hepatic lipidosis and immune cell infiltration. Our new mouse model and single-cell analysis provide a powerful resource to enable the discovery of previously unidentified senescence functions in vivo.
中文翻译:

p16 报告小鼠的生成及其在体内表征和靶向 p16high 细胞的用途。
细胞衰老在与年龄相关的器官功能障碍中起关键作用,但体内发病机制尚不清楚。在这里,我们生成了 p16-Cre ERT2 -tdTomato 小鼠模型,以在单细胞水平上分析p16高细胞的体内特征。我们发现在所有器官中均可检测到tdTomato 阳性 p16高细胞,这些细胞随着年龄的增长而富集。我们还发现这些细胞无法增殖,半衰期为 2.6 至 4.2 个月,具体取决于检查的组织。肝脏和肾脏中的单细胞转录组学显示 p16高细胞存在于各种细胞类型中,尽管在肝内皮和肾近端和远端小管上皮中占主导地位,并且这些细胞表现出异质的衰老相关表型。此外,消除 p16高细胞可改善非酒精性脂肪性肝炎相关的肝脏脂质沉积和免疫细胞浸润。我们的新小鼠模型和单细胞分析提供了强大的资源,可以在体内发现以前未识别的衰老功能。































 京公网安备 11010802027423号
京公网安备 11010802027423号