当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of Reaction Kinetics for Removal of NOx by Ozone and Hydrogen Peroxide
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.iecr.0c03820 Snigdha Khuntia 1 , Manish Kumar Sinha 2 , Gokulesh Mohan 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.iecr.0c03820 Snigdha Khuntia 1 , Manish Kumar Sinha 2 , Gokulesh Mohan 1
Affiliation
|
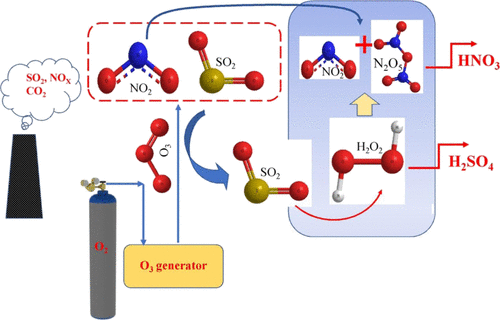
In this article, the kinetics of the ozone and hydrogen peroxide-based NO2 removal from flue gas has been evaluated using the well-known two-film theory. The effectiveness of the rate of absorption of NO2 has been tested by the effect of pH, temperature, H2O2 concentration, and so forth. This study also includes the comparison of the Hatta number (Ha) at various experimental conditions. It was observed that the reaction of NO2 is first order in nature with both ozone and hydrogen peroxide. The effect of SO2 concentration on the absorption of NO2 was negligible. However, the absorption efficiency decreases in the presence of higher SO2 concentration in the peroxone process. The predicted model shows a good fit with the experimental results for various NO2 inlet concentrations. The optimum pH for NO2 absorption for ozonation and peroxone processes was 8. At 318 K, absorption of NO2 shows the maximum value for the ozonation process, whereas for the peroxone process, it was 328 K. The effect of H2O2 on the absorption of NO2 in the aqueous phase was prominent with high Ha values. It was observed that NO2 absorption increases rapidly when H2O2 concentration is increased from 0 to 0.3 mol·L–1. In the zone of 0.5–3.5 mol·L–1 H2O2 concentration, the rate of NO2 absorption was slow.
中文翻译:

臭氧和过氧化氢去除NO x的反应动力学评价
在本文中,已经使用众所周知的两膜理论评估了从烟气中去除基于臭氧和过氧化氢的NO 2的动力学。已经通过pH,温度,H 2 O 2浓度等的影响来测试NO 2吸收速率的有效性。这项研究还包括在各种实验条件下的哈达数(Ha)的比较。观察到,NO 2与臭氧和过氧化氢的反应本质上都是一级反应。SO 2浓度对NO 2吸收的影响可忽略不计。然而,在较高的SO 2存在下吸收效率降低过氧化物处理过程中的浓度。预测的模型显示出与各种NO 2入口浓度的实验结果非常吻合的结果。臭氧化和过氧化物处理过程中吸收NO 2的最佳pH为8。在318 K时,臭氧化处理过程中NO 2的吸收显示最大值,而对于过氧化物处理,则为328K。H 2 O 2的影响水相中NO 2的吸收具有明显的高Ha值。观察到,当H 2 O 2浓度从0增加到0.3mol·L –1时,NO 2吸收迅速增加。。在浓度为0.5–3.5mol·L –1 H 2 O 2的区域中,NO 2的吸收速度较慢。
更新日期:2020-10-07
中文翻译:

臭氧和过氧化氢去除NO x的反应动力学评价
在本文中,已经使用众所周知的两膜理论评估了从烟气中去除基于臭氧和过氧化氢的NO 2的动力学。已经通过pH,温度,H 2 O 2浓度等的影响来测试NO 2吸收速率的有效性。这项研究还包括在各种实验条件下的哈达数(Ha)的比较。观察到,NO 2与臭氧和过氧化氢的反应本质上都是一级反应。SO 2浓度对NO 2吸收的影响可忽略不计。然而,在较高的SO 2存在下吸收效率降低过氧化物处理过程中的浓度。预测的模型显示出与各种NO 2入口浓度的实验结果非常吻合的结果。臭氧化和过氧化物处理过程中吸收NO 2的最佳pH为8。在318 K时,臭氧化处理过程中NO 2的吸收显示最大值,而对于过氧化物处理,则为328K。H 2 O 2的影响水相中NO 2的吸收具有明显的高Ha值。观察到,当H 2 O 2浓度从0增加到0.3mol·L –1时,NO 2吸收迅速增加。。在浓度为0.5–3.5mol·L –1 H 2 O 2的区域中,NO 2的吸收速度较慢。

































 京公网安备 11010802027423号
京公网安备 11010802027423号