当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural investigation of heteroyohimbine alkaloid synthesis reveals active site elements that control stereoselectivity.
Nature Communications ( IF 14.7 ) Pub Date : 2016-07-15 , DOI: 10.1038/ncomms12116 Anna Stavrinides 1 , Evangelos C Tatsis 1 , Lorenzo Caputi 1 , Emilien Foureau 2 , Clare E M Stevenson 1 , David M Lawson 1 , Vincent Courdavault 2 , Sarah E O'Connor 1
Nature Communications ( IF 14.7 ) Pub Date : 2016-07-15 , DOI: 10.1038/ncomms12116 Anna Stavrinides 1 , Evangelos C Tatsis 1 , Lorenzo Caputi 1 , Emilien Foureau 2 , Clare E M Stevenson 1 , David M Lawson 1 , Vincent Courdavault 2 , Sarah E O'Connor 1
Affiliation
|
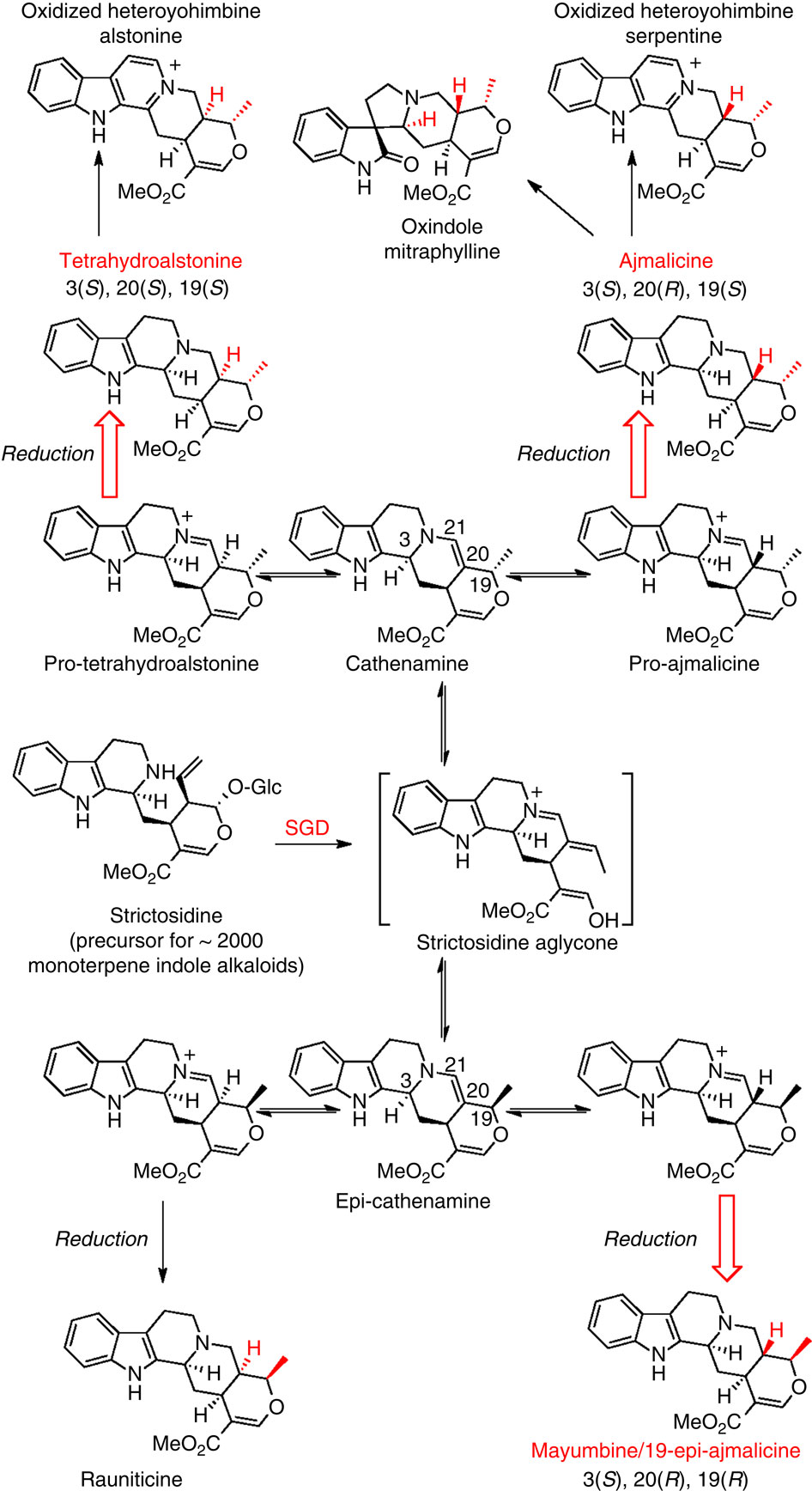
Plants produce an enormous array of biologically active metabolites, often with stereochemical variations on the same molecular scaffold. These changes in stereochemistry dramatically impact biological activity. Notably, the stereoisomers of the heteroyohimbine alkaloids show diverse pharmacological activities. We reported a medium chain dehydrogenase/reductase (MDR) from Catharanthus roseus that catalyses formation of a heteroyohimbine isomer. Here we report the discovery of additional heteroyohimbine synthases (HYSs), one of which produces a mixture of diastereomers. The crystal structures for three HYSs have been solved, providing insight into the mechanism of reactivity and stereoselectivity, with mutation of one loop transforming product specificity. Localization and gene silencing experiments provide a basis for understanding the function of these enzymes in vivo. This work sets the stage to explore how MDRs evolved to generate structural and biological diversity in specialized plant metabolism and opens the possibility for metabolic engineering of new compounds based on this scaffold.
中文翻译:

异育亨宾生物碱合成的结构研究揭示了控制立体选择性的活性位点元素。
植物产生大量具有生物活性的代谢物,通常在同一分子支架上具有立体化学变化。立体化学的这些变化极大地影响了生物活性。值得注意的是,异育亨宾生物碱的立体异构体显示出不同的药理活性。我们报道了一种来自长春花的中链脱氢酶/还原酶 (MDR),它催化异育亨宾异构体的形成。在这里,我们报告了额外的异育亨宾合酶 (HYS) 的发现,其中一种产生了非对映异构体的混合物。三个 HYS 的晶体结构已经得到解决,提供了对反应性和立体选择性机制的深入了解,其中一个环的突变转化了产物特异性。定位和基因沉默实验为了解这些酶在体内的功能提供了基础。这项工作为探索 MDR 如何进化以在专门的植物代谢中产生结构和生物多样性奠定了基础,并为基于该支架的新化合物的代谢工程开辟了可能性。
更新日期:2016-07-18
中文翻译:

异育亨宾生物碱合成的结构研究揭示了控制立体选择性的活性位点元素。
植物产生大量具有生物活性的代谢物,通常在同一分子支架上具有立体化学变化。立体化学的这些变化极大地影响了生物活性。值得注意的是,异育亨宾生物碱的立体异构体显示出不同的药理活性。我们报道了一种来自长春花的中链脱氢酶/还原酶 (MDR),它催化异育亨宾异构体的形成。在这里,我们报告了额外的异育亨宾合酶 (HYS) 的发现,其中一种产生了非对映异构体的混合物。三个 HYS 的晶体结构已经得到解决,提供了对反应性和立体选择性机制的深入了解,其中一个环的突变转化了产物特异性。定位和基因沉默实验为了解这些酶在体内的功能提供了基础。这项工作为探索 MDR 如何进化以在专门的植物代谢中产生结构和生物多样性奠定了基础,并为基于该支架的新化合物的代谢工程开辟了可能性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号