当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthetic Fluorinated L-Fucose Analogs Inhibit Proliferation of Cancer Cells and Primary Endothelial Cells.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-09-15 , DOI: 10.1021/acschembio.0c00228 Yuanwei Dai 1 , Ruth Hartke 2 , Chao Li 1 , Qiang Yang 1 , Jun O Liu 2 , Lai-Xi Wang 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-09-15 , DOI: 10.1021/acschembio.0c00228 Yuanwei Dai 1 , Ruth Hartke 2 , Chao Li 1 , Qiang Yang 1 , Jun O Liu 2 , Lai-Xi Wang 1
Affiliation
|
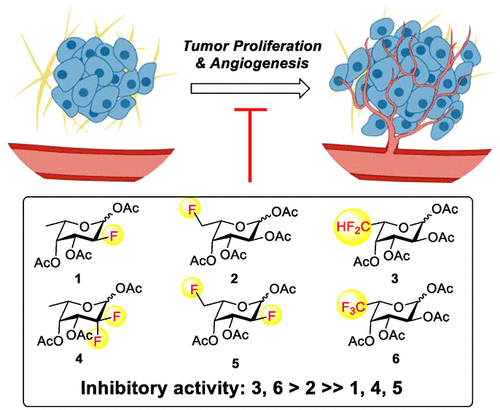
Fucosylation is one of the most prevalent modifications on N- and O-glycans of glycoproteins, and it plays an important role in various cellular processes and diseases. Small molecule inhibitors of fucosylation have shown promise as therapeutic agents for sickle cell disease, arthritis, and cancer. We describe here the design and synthesis of a panel of fluorinated l-fucose analogs bearing fluorine atoms at the C2 and/or C6 positions of l-fucose as metabolic fucosylation inhibitors. Preliminary study of their effects on cell proliferation revealed that the 6,6-difluoro-l-fucose (3) and 6,6,6-trifluoro-l-fucose (6) showed significant inhibitory activity against proliferation of human colon cancer cells and human umbilical vein endothelial cells. In contrast, the previously reported 2-deoxy-2-fluoro-l-fucose (1) had no apparent effects on proliferations of all the cell lines tested. To understand the mechanism of cell proliferation inhibition by the fluorinated l-fucose analogs, we performed chemoenzymatic synthesis of the corresponding GDP-fluorinated l-fucose analogs and tested their inhibitory activities against the mammalian α1,6-fucosyltransferase (FUT8). Interestingly, the corresponding GDP derivatives of 6,6-difluoro-l-fucose (3) and 6,6,6-trifluoro-l-fucose (6), which are the stronger proliferation inhibitors, showed much weaker inhibitory activity against FUT8 than that of the 2-deoxy-2-fluoro-l-fucose (1). These results suggest that FUT8 is not the major target of the 6-fluorinated fucose analogs (3 and 6). Instead, other factors, such as the key enzymes involved in the de novo GDP-fucose biosynthetic pathway and/or other fucosyltransferases involved in the biosynthesis of tumor-associated glyco-epitopes are most likely the targets of the fluorinated l-fucose analogs to achieve cell proliferation inhibition. To our knowledge, this is the first comparative study of various fluorinated l-fucose analogs for suppressing the proliferation of human cancer and primary endothelial cells required for angiogenesis.
中文翻译:

合成氟化 L-岩藻糖类似物可抑制癌细胞和原代内皮细胞的增殖。
岩藻糖基化是糖蛋白 N- 和 O- 聚糖最常见的修饰之一,在各种细胞过程和疾病中发挥着重要作用。岩藻糖基化的小分子抑制剂已显示出作为镰状细胞病、关节炎和癌症的治疗剂的前景。我们在此描述了一组在L-岩藻糖的 C2 和/或 C6 位点带有氟原子的氟化L-岩藻糖类似物作为代谢岩藻糖基化抑制剂的设计和合成。对细胞增殖影响的初步研究表明,6,6-二氟-l-岩藻糖 ( 3 ) 和 6,6,6-三氟-l-岩藻糖 ( 6 ) 对人结肠癌细胞的增殖具有显着的抑制活性,并且人脐静脉内皮细胞。相反,先前报道的2-脱氧-2-氟-l-岩藻糖( 1 )对所有测试的细胞系的增殖没有明显影响。为了了解氟化l-岩藻糖类似物抑制细胞增殖的机制,我们对相应的GDP-氟化l-岩藻糖类似物进行了化学酶法合成,并测试了它们对哺乳动物α1,6-岩藻糖基转移酶(FUT8)的抑制活性。有趣的是,6,6-二氟-l-岩藻糖 ( 3 ) 和 6,6,6-三氟-l-岩藻糖 ( 6 ) 相应的 GDP 衍生物是更强的增殖抑制剂,但对 FUT8 的抑制活性比 FUT8 弱得多。 2-脱氧-2-氟-1-岩藻糖( 1 )的结构。 这些结果表明 FUT8 不是 6-氟化岩藻糖类似物的主要靶标( 3和6 )。相反,其他因素,例如参与从头GDP-岩藻糖生物合成途径的关键酶和/或参与肿瘤相关糖表位生物合成的其他岩藻糖基转移酶,最有可能是氟化l-岩藻糖类似物实现目标。细胞增殖抑制。据我们所知,这是首次对各种氟化l-岩藻糖类似物抑制人类癌症和血管生成所需的原代内皮细胞增殖的比较研究。
更新日期:2020-10-17
中文翻译:

合成氟化 L-岩藻糖类似物可抑制癌细胞和原代内皮细胞的增殖。
岩藻糖基化是糖蛋白 N- 和 O- 聚糖最常见的修饰之一,在各种细胞过程和疾病中发挥着重要作用。岩藻糖基化的小分子抑制剂已显示出作为镰状细胞病、关节炎和癌症的治疗剂的前景。我们在此描述了一组在L-岩藻糖的 C2 和/或 C6 位点带有氟原子的氟化L-岩藻糖类似物作为代谢岩藻糖基化抑制剂的设计和合成。对细胞增殖影响的初步研究表明,6,6-二氟-l-岩藻糖 ( 3 ) 和 6,6,6-三氟-l-岩藻糖 ( 6 ) 对人结肠癌细胞的增殖具有显着的抑制活性,并且人脐静脉内皮细胞。相反,先前报道的2-脱氧-2-氟-l-岩藻糖( 1 )对所有测试的细胞系的增殖没有明显影响。为了了解氟化l-岩藻糖类似物抑制细胞增殖的机制,我们对相应的GDP-氟化l-岩藻糖类似物进行了化学酶法合成,并测试了它们对哺乳动物α1,6-岩藻糖基转移酶(FUT8)的抑制活性。有趣的是,6,6-二氟-l-岩藻糖 ( 3 ) 和 6,6,6-三氟-l-岩藻糖 ( 6 ) 相应的 GDP 衍生物是更强的增殖抑制剂,但对 FUT8 的抑制活性比 FUT8 弱得多。 2-脱氧-2-氟-1-岩藻糖( 1 )的结构。 这些结果表明 FUT8 不是 6-氟化岩藻糖类似物的主要靶标( 3和6 )。相反,其他因素,例如参与从头GDP-岩藻糖生物合成途径的关键酶和/或参与肿瘤相关糖表位生物合成的其他岩藻糖基转移酶,最有可能是氟化l-岩藻糖类似物实现目标。细胞增殖抑制。据我们所知,这是首次对各种氟化l-岩藻糖类似物抑制人类癌症和血管生成所需的原代内皮细胞增殖的比较研究。

































 京公网安备 11010802027423号
京公网安备 11010802027423号