当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spontaneous Formation of Asymmetric Oxygen Vacancies in Transition-Metal-Doped CeO2 Nanorods with Improved Activity for Carbonyl Sulfide Hydrolysis
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-09-14 , DOI: 10.1021/acscatal.0c02832 Shunzheng Zhao 1, 2 , Dongjuan Kang 1 , Yunpeng Liu 3 , Yanfeng Wen 1 , Xizhou Xie 1 , Honghong Yi 1, 2 , Xiaolong Tang 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-09-14 , DOI: 10.1021/acscatal.0c02832 Shunzheng Zhao 1, 2 , Dongjuan Kang 1 , Yunpeng Liu 3 , Yanfeng Wen 1 , Xizhou Xie 1 , Honghong Yi 1, 2 , Xiaolong Tang 1, 2
Affiliation
|
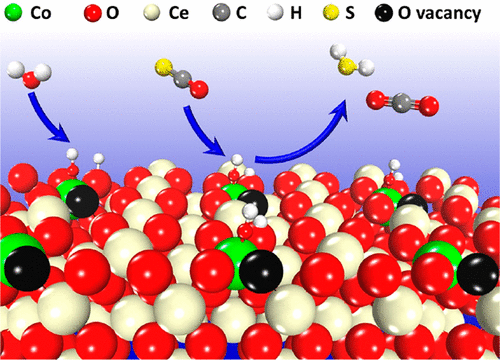
Introducing oxygen vacancies into metal oxides is a promising strategy to promote their catalytic activity, which has been extensively studied in heterogeneous catalysis. Herein, transition metal (M = Fe, Co, and Ni) doping was used to introduce oxygen vacancies in CeO2 and promote activity for carbonyl sulfide (COS) hydrolysis. Various techniques were performed to accurately characterize the catalyst structure and state. The transition metals successfully entered the crystal lattice of CeO2 and formed a solid solution structure. The metal-doped CeO2 (M/CeO2) showed improved reduction properties, more Ce3+ and oxygen vacancies in comparison with pure CeO2. The introduction of transition metal greatly enhanced activity of M/CeO2 for COS hydrolysis. Among them, the Co/CeO2 sample displayed the highest activity and H2S selectivity. The roles of metal doping in improving activity were explored on the basis of DFT calculations. The strong interaction between doped metals and CeO2 promotes the spontaneous formation of asymmetric oxygen vacancies in M/CeO2. These asymmetric oxygen vacancies facilitate the activation and dissociation of H2O and generation of active hydroxyls, which contributes to the enhanced activity for COS hydrolysis. This work provides an attractive method for obtaining nonprecious metal catalysts for COS hydrolysis.
中文翻译:

过渡金属掺杂的CeO 2纳米棒中自发形成不对称氧空位,具有改进的羰基硫水解活性。
将氧空位引入金属氧化物是提高其催化活性的一种有前途的策略,已在多相催化中进行了广泛研究。此处,过渡金属(M = Fe,Co和Ni)掺杂用于在CeO 2中引入氧空位并提高羰基硫(COS)水解的活性。进行了各种技术以准确表征催化剂的结构和状态。过渡金属成功地进入CeO 2的晶格并形成固溶体结构。与纯CeO 2相比,金属掺杂的CeO 2(M / CeO 2)表现出改善的还原性能,更多的Ce 3+和氧空位。过渡金属的引入大大提高了M / CeO 2的COS水解活性。其中,Co / CeO 2样品显示出最高的活性和H 2 S的选择性。在DFT计算的基础上,探讨了金属掺杂在提高活性中的作用。掺杂金属与CeO 2之间的强相互作用促进了M / CeO 2中不对称氧空位的自发形成。这些不对称的氧空位促进了H 2 O的活化和解离以及活性羟基的产生,这有助于提高COS水解的活性。这项工作为获得用于COS水解的非贵金属催化剂提供了一种有吸引力的方法。
更新日期:2020-10-17
中文翻译:

过渡金属掺杂的CeO 2纳米棒中自发形成不对称氧空位,具有改进的羰基硫水解活性。
将氧空位引入金属氧化物是提高其催化活性的一种有前途的策略,已在多相催化中进行了广泛研究。此处,过渡金属(M = Fe,Co和Ni)掺杂用于在CeO 2中引入氧空位并提高羰基硫(COS)水解的活性。进行了各种技术以准确表征催化剂的结构和状态。过渡金属成功地进入CeO 2的晶格并形成固溶体结构。与纯CeO 2相比,金属掺杂的CeO 2(M / CeO 2)表现出改善的还原性能,更多的Ce 3+和氧空位。过渡金属的引入大大提高了M / CeO 2的COS水解活性。其中,Co / CeO 2样品显示出最高的活性和H 2 S的选择性。在DFT计算的基础上,探讨了金属掺杂在提高活性中的作用。掺杂金属与CeO 2之间的强相互作用促进了M / CeO 2中不对称氧空位的自发形成。这些不对称的氧空位促进了H 2 O的活化和解离以及活性羟基的产生,这有助于提高COS水解的活性。这项工作为获得用于COS水解的非贵金属催化剂提供了一种有吸引力的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号