当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly Selective CO2 Electroreduction to CH4 by in situ Generated Cu2O Single-Type Sites on Conductive MOF: Stabilizing Key Intermediates with Hydrogen Bond.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-14 , DOI: 10.1002/anie.202010601 Jun-Dong Yi 1 , Ruikuan Xie 1 , Zai-Lai Xie 2 , Guo-Liang Chai 1, 3 , Tian-Fu Liu 1, 3 , Rui-Ping Chen 1 , Yuan-Biao Huang 1, 3 , Rong Cao 1, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-14 , DOI: 10.1002/anie.202010601 Jun-Dong Yi 1 , Ruikuan Xie 1 , Zai-Lai Xie 2 , Guo-Liang Chai 1, 3 , Tian-Fu Liu 1, 3 , Rui-Ping Chen 1 , Yuan-Biao Huang 1, 3 , Rong Cao 1, 3
Affiliation
|
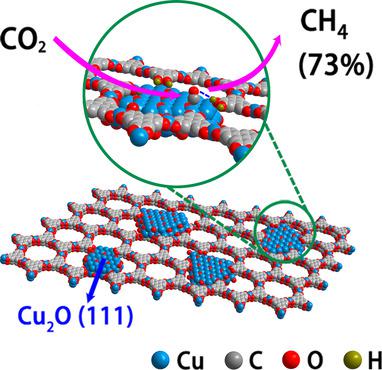
It is still a great challenge to achieve high selectivity of CH4 in CO2 electroreduction reactions (CO2RR) because of the similar reduction potentials of possible products and the sluggish kinetics for CO2 activation. Stabilizing key reaction intermediates by single type of active sites supported on porous conductive material is crucial to achieve high selectivity for single product such as CH4. Here, Cu2O(111) quantum dots with an average size of 3.5 nm are in situ synthesized on a porous conductive copper‐based metal–organic framework (CuHHTP), exhibiting high selectivity of 73 % towards CH4 with partial current density of 10.8 mA cm−2 at −1.4 V vs. RHE (reversible hydrogen electrode) in CO2RR. Operando infrared spectroscopy and DFT calculations reveal that the key intermediates (such as *CH2O and *OCH3) involved in the pathway of CH4 formation are stabilized by the single active Cu2O(111) and hydrogen bonding, thus generating CH4 instead of CO.
中文翻译:

通过在导电MOF上原位生成的Cu2O单型位点,将高选择性的CO2电还原为CH4:稳定具有氢键的关键中间体。
在CO 2电还原反应(CH 2 RR)中实现CH 4的高选择性仍然是一个巨大的挑战,因为可能的产物具有相似的还原电势,并且活化CO 2的反应动力学缓慢。通过负载在多孔导电材料上的单一类型的活性位点来稳定关键反应中间体,对于获得单一产品(例如CH 4)的高选择性至关重要。在此,在多孔导电铜基金属有机框架(CuHHTP)上原位合成平均尺寸为3.5 nm的Cu 2 O(111)量子点,对CH 4表现出73%的高选择性,且部分电流密度为10.8 mA厘米-2在-1.4 V相对于CO 2 RR中的RHE(可逆氢电极)的情况。Operando红外光谱和DFT计算表明,CH 4形成途径中涉及的关键中间体(例如* CH 2 O和* OCH 3)通过单个活性Cu 2 O(111)和氢键得以稳定,从而生成CH 4代替CO。
更新日期:2020-09-14
中文翻译:

通过在导电MOF上原位生成的Cu2O单型位点,将高选择性的CO2电还原为CH4:稳定具有氢键的关键中间体。
在CO 2电还原反应(CH 2 RR)中实现CH 4的高选择性仍然是一个巨大的挑战,因为可能的产物具有相似的还原电势,并且活化CO 2的反应动力学缓慢。通过负载在多孔导电材料上的单一类型的活性位点来稳定关键反应中间体,对于获得单一产品(例如CH 4)的高选择性至关重要。在此,在多孔导电铜基金属有机框架(CuHHTP)上原位合成平均尺寸为3.5 nm的Cu 2 O(111)量子点,对CH 4表现出73%的高选择性,且部分电流密度为10.8 mA厘米-2在-1.4 V相对于CO 2 RR中的RHE(可逆氢电极)的情况。Operando红外光谱和DFT计算表明,CH 4形成途径中涉及的关键中间体(例如* CH 2 O和* OCH 3)通过单个活性Cu 2 O(111)和氢键得以稳定,从而生成CH 4代替CO。














































 京公网安备 11010802027423号
京公网安备 11010802027423号