Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2020-09-14 , DOI: 10.1016/j.comptc.2020.113029 Ye Zhang , Guoqing Chen , Jiao Gu , Chaoqun Ma , Lei Li , Chun Zhu , Hui Gao , Chengwei Wang , Yunpeng Shang , Zichen Yang
|
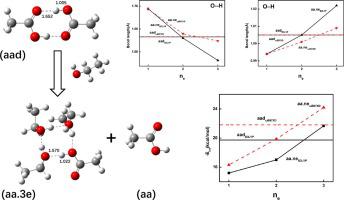
Electronic structure calculations are applied on acetic acid monomers, dimers and clusters in water and ethanol solvent. Acetic acid is normally existed as dimeric structure in gas, vapor and liquid phase. It is predicted that sufficient ethanol molecules can dissociate this dimeric structure, while stable clusters can be formed by acetic acid units and ethanol units. To investigate this process, optimized structures, geometry parameters, interaction energy values and simulated Raman spectra of acetic acid dimers and clusters are discussed. Geometry parameters indicates that at least three ethanol molecules can break the dimeric structures, while just two H2O molecules are needed to dissociate acetic acid dimer. Interaction energy parameters justify the minimum number of solvent molecules to break dimeric structures further. Energy decomposition analysis of interaction suggests that hydrogen-bond interaction plays a central role in interaction and acetic acid clusters with ethanol units have higher value of electrostatic energy than that of hydrated clusters, which causes that more ethanol molecules are needed to break acetic acid dimer than water molecules. Reduced density gradient function analysis investigates the position and strength of H-bond interaction. The red shifts of the O H peak position of theoretical spectra for acetic acid clusters imply the weakening of O
H peak position of theoretical spectra for acetic acid clusters imply the weakening of O H stretching and the strengthen of H-bond interaction while clusters’ size enlarging. Experimental Raman spectra justify the result of theoretical spectra.
H stretching and the strengthen of H-bond interaction while clusters’ size enlarging. Experimental Raman spectra justify the result of theoretical spectra.
中文翻译:

乙酸在乙醇溶液中二聚和离解的理论研究
电子结构计算适用于水和乙醇溶剂中的乙酸单体,二聚体和簇。乙酸通常以二聚物结构形式存在于气相,气相和液相中。据预测,足够的乙醇分子可以解离该二聚体结构,而稳定的簇可以由乙酸单元和乙醇单元形成。为了研究这一过程,讨论了乙酸二聚体和团簇的优化结构,几何参数,相互作用能值和模拟拉曼光谱。几何参数表明,至少三个乙醇分子可以破坏二聚体结构,而只有两个H 2需要O分子解离乙酸二聚体。相互作用能参数证明了最少数量的溶剂分子可以进一步破坏二聚体结构。相互作用的能量分解分析表明,氢键相互作用在相互作用中起着核心作用,带有乙醇单元的乙酸簇比水合簇具有更高的静电能值,这导致需要更多的乙醇分子来分解乙酸二聚体。水分子。降低密度梯度函数分析研究了氢键相互作用的位置和强度。 乙酸簇理论光谱的O H峰位置的红移表明O的减弱
乙酸簇理论光谱的O H峰位置的红移表明O的减弱 团簇尺寸增大时,H伸展并增强了H键相互作用。实验拉曼光谱证明理论光谱的结果是正确的。
团簇尺寸增大时,H伸展并增强了H键相互作用。实验拉曼光谱证明理论光谱的结果是正确的。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号