Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Perfluorocarbon@Porphyrin Nanoparticles for Tumor Hypoxia Relief to Enhance Photodynamic Therapy against Liver Metastasis of Colon Cancer.
ACS Nano ( IF 15.8 ) Pub Date : 2020-09-11 , DOI: 10.1021/acsnano.0c05617
Xiaolong Liang 1 , Min Chen 2 , Pravin Bhattarai 2 , Sadaf Hameed 2 , Zhifei Dai 2
ACS Nano ( IF 15.8 ) Pub Date : 2020-09-11 , DOI: 10.1021/acsnano.0c05617
Xiaolong Liang 1 , Min Chen 2 , Pravin Bhattarai 2 , Sadaf Hameed 2 , Zhifei Dai 2
Affiliation
|
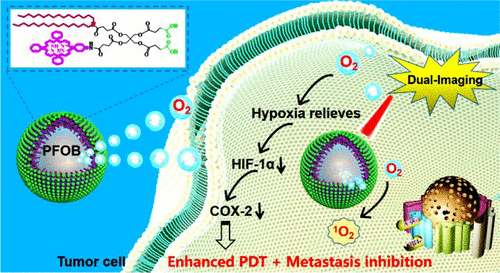
Photodynamic therapy (PDT) shows great promise for the treatment of colon cancer. However, practically, it is a great challenge to use a nanocarrier for the codelivery of both the photosensitizer and oxygen to improve PDT against PDT-induced hypoxia, which is closely related to tumor metastasis. Hence, an effective strategy was proposed to develop an oxygen self-supplemented PDT nanocarrier based on the ultrasonic dispersion of perfluorooctyl bromide (PFOB) liquid into the preformed porphyrin grafted lipid (PGL) nanoparticles (NPs) with high porphyrin loading content of 38.5%, followed by entrapping oxygen. Interestingly, the orderly arranging mode of porphyrins and alkyl chains in PGL NPs not only guarantees a high efficacy of singlet oxygen generation but also reduces fluorescence loss of porphyrins to enable PGL NPs to be highly fluorescent. More importantly, PFOB liquid was stabilized inside PGL NPs with an ultrahigh loading content of 98.15% due to the strong hydrophobic interaction between PGL and PFOB molecules, facilitating efficient oxygen delivery. Both in vitro and in vivo results demonstrated that the obtained O2@PFOB@PGL NPs could act as a prominent oxygen reservoir and effectively replenish oxygen into the hypoxic tumors with no need for external stimulation, conducive to augmented singlet oxygen generation, hypoxia relief, and subsequent downregulation of COX-2 expression. As a result, the use of O2@PFOB@PGL NPs for hypoxia relief dramatically inhibits tumor growth and liver metastasis in an HT-29 colon cancer mouse model. In addition, the O2@PFOB@PGL NPs could serve as a bimodal contrast agent to enhance fluorescence and CT imaging, visualizing nanoparticle accumulation to guide the subsequent laser irradiation for precise PDT.
中文翻译:

全氟化碳@卟啉纳米颗粒用于缓解肿瘤缺氧,以增强抗结肠癌肝转移的光动力疗法。
光动力疗法(PDT)显示了治疗结肠癌的巨大希望。然而,实际上,将纳米载体用于光敏剂和氧气的代码传递对改善PDT抵抗PDT诱导的缺氧是一个巨大的挑战,PDT诱导的缺氧与肿瘤转移密切相关。因此,基于全氟辛基溴(PFOB)液体在预先形成的卟啉接枝脂质(PGL)纳米颗粒(NPs)中的超声分散,提出了一种有效的策略来开发一种氧自补给的PDT纳米载体,该载体具有38.5%的高卟啉含量,然后夹带氧气。有趣的是,PGL NP中卟啉和烷基链的有序排列方式不仅保证了单线态氧产生的高效率,而且减少了卟啉的荧光损失,从而使PGL NP具有高荧光性。更重要的是,由于PGL和PFOB分子之间的强疏水作用,PFOB液体被稳定在PGL NP内部,具有98.15%的超高负载量,从而促进了有效的氧气输送。都体外和体内结果表明,所获得的O 2 @ PFOB @ PGL NPs可以作为重要的氧气库,无需外部刺激即可有效地将氧气补充到缺氧肿瘤中,有利于增加单线态氧的产生,减少缺氧和随后下调COX-2表达。因此,在HT-29结肠癌小鼠模型中,使用O 2 @ PFOB @ PGL NP缓解缺氧会显着抑制肿瘤生长和肝转移。此外,O 2 @ PFOB @ PGL NP可以用作双峰造影剂,以增强荧光和CT成像,可视化纳米颗粒的积累,以指导随后的激光照射以实现精确的PDT。
更新日期:2020-09-11
中文翻译:

全氟化碳@卟啉纳米颗粒用于缓解肿瘤缺氧,以增强抗结肠癌肝转移的光动力疗法。
光动力疗法(PDT)显示了治疗结肠癌的巨大希望。然而,实际上,将纳米载体用于光敏剂和氧气的代码传递对改善PDT抵抗PDT诱导的缺氧是一个巨大的挑战,PDT诱导的缺氧与肿瘤转移密切相关。因此,基于全氟辛基溴(PFOB)液体在预先形成的卟啉接枝脂质(PGL)纳米颗粒(NPs)中的超声分散,提出了一种有效的策略来开发一种氧自补给的PDT纳米载体,该载体具有38.5%的高卟啉含量,然后夹带氧气。有趣的是,PGL NP中卟啉和烷基链的有序排列方式不仅保证了单线态氧产生的高效率,而且减少了卟啉的荧光损失,从而使PGL NP具有高荧光性。更重要的是,由于PGL和PFOB分子之间的强疏水作用,PFOB液体被稳定在PGL NP内部,具有98.15%的超高负载量,从而促进了有效的氧气输送。都体外和体内结果表明,所获得的O 2 @ PFOB @ PGL NPs可以作为重要的氧气库,无需外部刺激即可有效地将氧气补充到缺氧肿瘤中,有利于增加单线态氧的产生,减少缺氧和随后下调COX-2表达。因此,在HT-29结肠癌小鼠模型中,使用O 2 @ PFOB @ PGL NP缓解缺氧会显着抑制肿瘤生长和肝转移。此外,O 2 @ PFOB @ PGL NP可以用作双峰造影剂,以增强荧光和CT成像,可视化纳米颗粒的积累,以指导随后的激光照射以实现精确的PDT。

































 京公网安备 11010802027423号
京公网安备 11010802027423号