当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, spectroscopic, DFT, HSA binding and docking studies of new 1,5-bis(4-chlorophenyl)-3-(2-(4-methylpiperazin-1-yl)quinolin-3-yl)pentane-1,5-dione
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129260 Arul Murugesan , Thishana Singh , Ramar Rajamanikandan , Madhan Vinu , Malaichamy Ilanchelian , Chia-Her Lin , Robert Moonsamy Gengan
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129260 Arul Murugesan , Thishana Singh , Ramar Rajamanikandan , Madhan Vinu , Malaichamy Ilanchelian , Chia-Her Lin , Robert Moonsamy Gengan
|
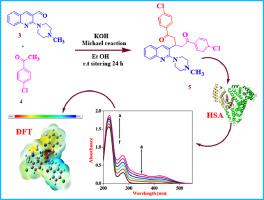
Abstract 1,5-Bis(4-chlorophenyl)-3-(2-(4-methylpiperazin-1-yl)quinolin-3-yl)pentane-1,5-dione was synthesised and characterised using single-crystal X-ray Crystallography, FT-IR, 1H-NMR, 13C-NMR and UV-Visible spectroscopy. DFT calculations were performed at the B3LYP/6-311++G (d.p) level of theory in the gas phase. Frontier Molecular Orbitals (FMO) yielded HOMO-LUMO energy as: EHOMO = -6.015 eV, ELUMO = -2.525 eV and energy gap, ΔEgap = 3.490 eV. Fukui Function Analysis (FFA) indicated the reactive sites for electrophilic, and nucleophilic attack. The molecule's electrophilic addition site is 4-N in the piperazine group with a value of 0.020. The site for nucleophilic attack is both 13-C and 15-C in the quinoline group with values of 0.02 and 0.031 respectively. The biological activity was elucidated by molecular docking studies that gave a ΔG value for HSA binding of -26.44 kJ mol−1 which is approximately similar to the experimental value obtained from emission spectral data of -32.15 kJ mol−1.
中文翻译:

新型 1,5-双(4-氯苯基)-3-(2-(4-甲基哌嗪-1-基)喹啉-3-基)戊烷-1,5-的合成、光谱、DFT、HSA结合和对接研究二酮
摘要 合成了 1,5-双(4-氯苯基)-3-(2-(4-甲基哌嗪-1-基)喹啉-3-基)戊烷-1,5-二酮,并使用单晶 X 射线进行表征。晶体学、FT-IR、1H-NMR、13C-NMR和紫外-可见光谱。DFT 计算是在 B3LYP/6-311++G (dp) 理论水平的气相中进行的。Frontier Molecular Orbitals (FMO) 产生的 HOMO-LUMO 能量为:EHOMO = -6.015 eV,ELUMO = -2.525 eV 和能隙,ΔEgap = 3.490 eV。Fukui 函数分析 (FFA) 表明亲电和亲核攻击的反应位点。该分子的亲电加成位点是哌嗪基团中的 4-N,值为 0.020。喹啉组中亲核攻击的位点是 13-C 和 15-C,其值分别为 0.02 和 0.031。
更新日期:2021-01-01
中文翻译:

新型 1,5-双(4-氯苯基)-3-(2-(4-甲基哌嗪-1-基)喹啉-3-基)戊烷-1,5-的合成、光谱、DFT、HSA结合和对接研究二酮
摘要 合成了 1,5-双(4-氯苯基)-3-(2-(4-甲基哌嗪-1-基)喹啉-3-基)戊烷-1,5-二酮,并使用单晶 X 射线进行表征。晶体学、FT-IR、1H-NMR、13C-NMR和紫外-可见光谱。DFT 计算是在 B3LYP/6-311++G (dp) 理论水平的气相中进行的。Frontier Molecular Orbitals (FMO) 产生的 HOMO-LUMO 能量为:EHOMO = -6.015 eV,ELUMO = -2.525 eV 和能隙,ΔEgap = 3.490 eV。Fukui 函数分析 (FFA) 表明亲电和亲核攻击的反应位点。该分子的亲电加成位点是哌嗪基团中的 4-N,值为 0.020。喹啉组中亲核攻击的位点是 13-C 和 15-C,其值分别为 0.02 和 0.031。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号