European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2020-09-12 , DOI: 10.1016/j.ejps.2020.105544 Francesca Ferlenghi 1 , Paola Maccioni 2 , Claudia Mugnaini 3 , Antonella Brizzi 3 , Federica Fara 2 , Rafaela Mostallino 4 , M Paola Castelli 4 , Giancarlo Colombo 2 , Marco Mor 1 , Federica Vacondio 1 , Federico Corelli 3
|
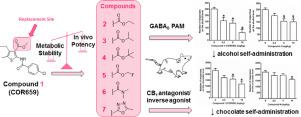
We report an in vitro phase I metabolism study on COR659 (1), a 2-acylaminothiophene derivative able to suppress alcohol and chocolate self-administration in rats, likely via positive allosteric modulation of the GABAB receptor and antagonism/inverse agonism at the cannabinoid CB1 receptor. Given the identification of the methyl ester group at C-3 of the thiophene ring as a metabolic soft spot, we also report the chemical optimization project aimed to balance metabolic stability with in vitro and in vivo potency on a set of 3-substituted COR659 analogues.
High performance liquid chromatography coupled to tandem and high resolution mass spectrometry was employed for the characterization of in vitro metabolism and in vivo pharmacokinetics of COR659 in rats. In vitro [35S]GTPγS binding assays on stimulated GABAB and CB1 receptors, in combination with alcohol and chocolate self-administration experiments in rats, were employed to assess the pharmacological profile of this novel set of analogues, using COR659 as reference compound.
Eight metabolites of COR659 were discovered in liver microsomal incubates; two of them (M1, M2) were identified by comparison with synthetic reference standards. M2, oxidation product of methyl group at C-5 of the thiophene ring, was a major metabolite in vitro, but showed a low systemic exposure in vivo. M1, cleavage product of the methyl ester group at C-3, revealed in vitro an unusual mechanism of metabolism by a NADPH-dependent route and, in vivo, it maintained high and persistent levels in plasma, which could represent a potential pharmacokinetic and toxicological issue.
In the novel set of COR659 analogues, those bearing branched alkyl substituents on the ester group, showed an improved in vitro metabolic stability (2–4), had an in vitro GABAB PAM (2–4) and/or CB1 partial agonist/antagonist profile (2–3) and maintained the ability to reduce alcohol (2–4) and/or chocolate (4) self-administration in rats. Both PK and PD data ruled out any involvement of metabolite M1 in the in vivo potency of COR659 and 4.
The present results, therefore, highlight the importance to design and synthesize novel compounds endowed with the dual activity profile and devoid of metabolic liabilities.
中文翻译:

GABA B受体阳性变构调节剂COR659:体外代谢,大鼠体内药代动力学,代谢保护衍生物的合成和药理学表征。
我们报告了一项对COR659(1)的体外I期代谢研究,COR659是一种2-酰基氨基噻吩衍生物,能够抑制大鼠酒精和巧克力的自我给药,可能是通过对GABA B受体的正变构调节和对大麻素的拮抗作用/反向激动作用CB 1受体。鉴于已将噻吩环C-3处的甲基酯基团鉴定为代谢弱点,我们还报告了化学优化项目,旨在平衡一组3取代COR659类似物在体内和体外的代谢稳定性与代谢稳定性。
高效液相色谱串联和高分辨率质谱用于表征大鼠COR659的体外代谢和体内药代动力学。体外[ 35 S] GTP γ S结合于刺激的GABA测定乙和CB 1点用酒精和巧克力自身给药实验的受体,结合在大鼠中,采用以评估这种新颖的组类似物的药理学特性,使用COR659作为参考化合物。
在肝微粒体温育中发现了COR659的八种代谢物;通过与合成参考标准品比较确定了其中的两个(M1,M2)。M2是噻吩环C-5处甲基的氧化产物,在体外是主要的代谢产物,但在体内显示出较低的全身暴露。M1是C-3处的甲酯基团的裂解产物,在体外揭示了NADPH依赖性途径的异常代谢机制,并且在体内,其在血浆中维持了高水平和持续水平,这可能代表了潜在的药代动力学和毒理学问题。
在一组新的COR659类似物中,那些在酯基团上带有分支烷基取代基的化合物显示出改善的体外代谢稳定性(2-4),具有体外GABA B PAM(2-4)和/或CB 1部分激动剂/拮抗剂(2-3)并保持减少大鼠酒精(2-4)和/或巧克力(4)自我给药的能力。PK和PD数据均排除了代谢物M1与COR659和4的体内效力有关。
因此,目前的结果强调了设计和合成具有双重活性特征且没有代谢负债的新型化合物的重要性。































 京公网安备 11010802027423号
京公网安备 11010802027423号