当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism and Nature of Active Sites for Methanol Synthesis from CO/CO2 on Cu/CeO2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-09-10 , DOI: 10.1021/acscatal.0c02909 Jiadong Zhu 1 , Yaqiong Su 1 , Jiachun Chai 1 , Valery Muravev 1 , Nikolay Kosinov 1 , Emiel J. M. Hensen 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-09-10 , DOI: 10.1021/acscatal.0c02909 Jiadong Zhu 1 , Yaqiong Su 1 , Jiachun Chai 1 , Valery Muravev 1 , Nikolay Kosinov 1 , Emiel J. M. Hensen 1
Affiliation
|
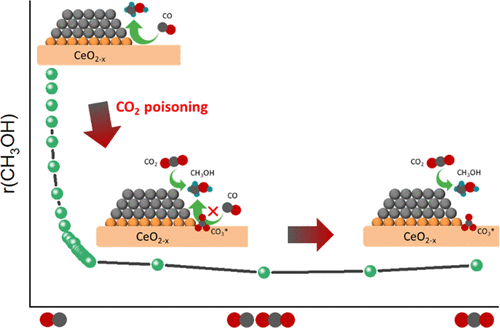
CO2 hydrogenation to methanol can play an important role in meeting the sustainability goals of the chemical industry. In this study, we investigated in detail the role of the Cu–CeO2 interactions for methanol synthesis, emphasizing the role of the copper surface and interface sites between copper and ceria for the hydrogenation of CO2 and CO. A combined CO2–N2O titration approach was developed to quantify the exposed metallic copper sites and ceria oxygen vacancies in reduced Cu/CeO2 catalysts. Extensive characterization shows that copper dispersion is strongly enhanced by strong Cu–CeO2 interactions in comparison to Cu/SiO2. CO2 hydrogenation activity data show that the Cu/CeO2 catalysts displayed higher methanol selectivity compared to a reference Cu/SiO2 catalyst. The improved methanol selectivity stems from inhibition of the reverse water-gas-shift activity. The role of CO in CO2-to-methanol conversion was studied by steady-state and transient cofeeding activity measurements together with (quasi) in situ characterization (TPH, XPS, SSITKA, and IR spectroscopy). The Cu–CeO2 interface provides active sites for the direct hydrogenation of CO to methanol via a formyl intermediate. Cofeeding of small amounts of CO2 to a CO/H2 mixture poisons these interfacial sites due to the formation of carbonate-like species. Methanol synthesis proceeds mainly via CO2 hydrogenation in which the metallic Cu surface provides the active sites.
中文翻译:

Cu / CeO 2上CO / CO 2合成甲醇的活性部位的机理和性质
将CO 2加氢成甲醇可在实现化学工业的可持续性目标中发挥重要作用。在这项研究中,我们详细研究在Cu-的CeO的作用2个相互作用甲醇合成,强调铜和氧化铈之间的铜表面和界面部位的作用,为CO的氢化2和CO。的组合CO 2 -N开发了2 O滴定法来量化还原的Cu / CeO 2催化剂中裸露的金属铜位和二氧化铈氧空位。广泛的特征表明,与Cu / SiO 2相比,Cu -CeO 2的强相互作用大大增强了铜的分散性。一氧化碳2氢化活性数据表明,与参比Cu / SiO 2催化剂相比,Cu / CeO 2催化剂显示出更高的甲醇选择性。改善的甲醇选择性源于对反向水煤气变换活性的抑制。通过稳态和瞬时共进料活性测量以及(近似)原位表征(TPH,XPS,SSITKA和IR光谱)研究了CO在CO 2转化为甲醇中的作用。Cu–CeO 2界面为通过甲酰基中间体将CO直接加氢为甲醇提供了活性部位。将少量的CO 2共进料到CO / H 2混合物由于形成碳酸盐样物质而使这些界面部位中毒。甲醇的合成主要通过CO 2氢化进行,其中金属Cu表面提供了活性位点。
更新日期:2020-10-02
中文翻译:

Cu / CeO 2上CO / CO 2合成甲醇的活性部位的机理和性质
将CO 2加氢成甲醇可在实现化学工业的可持续性目标中发挥重要作用。在这项研究中,我们详细研究在Cu-的CeO的作用2个相互作用甲醇合成,强调铜和氧化铈之间的铜表面和界面部位的作用,为CO的氢化2和CO。的组合CO 2 -N开发了2 O滴定法来量化还原的Cu / CeO 2催化剂中裸露的金属铜位和二氧化铈氧空位。广泛的特征表明,与Cu / SiO 2相比,Cu -CeO 2的强相互作用大大增强了铜的分散性。一氧化碳2氢化活性数据表明,与参比Cu / SiO 2催化剂相比,Cu / CeO 2催化剂显示出更高的甲醇选择性。改善的甲醇选择性源于对反向水煤气变换活性的抑制。通过稳态和瞬时共进料活性测量以及(近似)原位表征(TPH,XPS,SSITKA和IR光谱)研究了CO在CO 2转化为甲醇中的作用。Cu–CeO 2界面为通过甲酰基中间体将CO直接加氢为甲醇提供了活性部位。将少量的CO 2共进料到CO / H 2混合物由于形成碳酸盐样物质而使这些界面部位中毒。甲醇的合成主要通过CO 2氢化进行,其中金属Cu表面提供了活性位点。















































 京公网安备 11010802027423号
京公网安备 11010802027423号