当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Size-Controlled Pd Nanoparticles Loaded on Co3O4 Nanoparticles by Calcination for Enhanced CO Oxidation
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-12-05 00:00:00 , DOI: 10.1021/acsanm.9b02056
Rui Huang 1 , Kyeounghak Kim 1 , Hyung Jun Kim 1 , Myeong Gon Jang 1 , Jeong Woo Han 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-12-05 00:00:00 , DOI: 10.1021/acsanm.9b02056
Rui Huang 1 , Kyeounghak Kim 1 , Hyung Jun Kim 1 , Myeong Gon Jang 1 , Jeong Woo Han 1
Affiliation
|
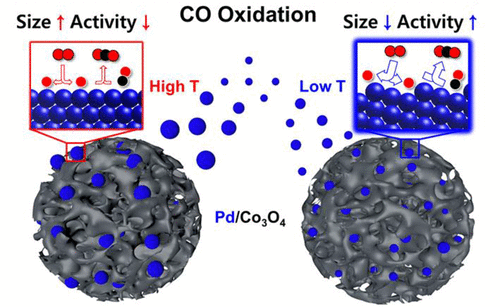
In accordance with Euro 6 emission standards, exhaust emissions are required to be substantially lowered especially via significant improvement in the efficiency of catalytic oxidation to reduce toxic carbon monoxide (CO). It has been reported that nanoparticles with high surface-to-volume ratio efficiently enhance the catalytic activity by providing additional active sites per unit area. However, the principle underlying this phenomenon is still not clear. To systematically elucidate the effect of metal nanoparticles on catalytic activity, we controlled the size of Pd nanoparticles loaded on Co3O4 by changing the calcination temperature. This approach was used to fine-tune the particle size from 2.5 to 10.6 nm. We found that Pd particle size is a dominant factor that affects the CO oxidation activity; smaller Pd particles yielded better catalytic activity. Three important reaction steps were identified through DFT calculations, based on which a series of temperature-programmed desorption and reduction measurements such as CO-TPD, CO chemisorption, O2-TPD, and CO-TPR were performed. The better abilities of CO desorption and O2 dissociation as well as the easier CO2 formation were found to be responsible for the higher activity of smaller Pd particles. We believe that our findings represent a potential strategy for the development of highly efficient catalysts.
中文翻译:

通过煅烧负载在Co 3 O 4纳米颗粒上的尺寸控制的Pd纳米颗粒可增强CO氧化
根据欧6排放标准,需要显着降低废气排放,尤其是通过显着提高催化氧化效率以减少有毒一氧化碳(CO)的排放。据报道,具有高的表面体积比的纳米颗粒通过在单位面积上提供额外的活性位点而有效地增强了催化活性。但是,这种现象的基本原理仍不清楚。为了系统地阐明金属纳米颗粒对催化活性的影响,我们控制了负载在Co 3 O 4上的Pd纳米颗粒的大小。通过改变煅烧温度。该方法用于将粒径从2.5 nm微调至10.6 nm。我们发现钯的粒径是影响CO氧化活性的主要因素。较小的Pd颗粒具有更好的催化活性。通过DFT计算确定了三个重要的反应步骤,在此基础上进行了一系列程序升温编程的解吸和还原测量,例如CO-TPD,CO化学吸附,O 2 -TPD和CO-TPR。CO解吸和O 2解离的能力越强,CO 2越容易发现形成Pd较小的Pd较高的活性。我们相信,我们的发现代表了开发高效催化剂的潜在策略。
更新日期:2019-12-05
中文翻译:

通过煅烧负载在Co 3 O 4纳米颗粒上的尺寸控制的Pd纳米颗粒可增强CO氧化
根据欧6排放标准,需要显着降低废气排放,尤其是通过显着提高催化氧化效率以减少有毒一氧化碳(CO)的排放。据报道,具有高的表面体积比的纳米颗粒通过在单位面积上提供额外的活性位点而有效地增强了催化活性。但是,这种现象的基本原理仍不清楚。为了系统地阐明金属纳米颗粒对催化活性的影响,我们控制了负载在Co 3 O 4上的Pd纳米颗粒的大小。通过改变煅烧温度。该方法用于将粒径从2.5 nm微调至10.6 nm。我们发现钯的粒径是影响CO氧化活性的主要因素。较小的Pd颗粒具有更好的催化活性。通过DFT计算确定了三个重要的反应步骤,在此基础上进行了一系列程序升温编程的解吸和还原测量,例如CO-TPD,CO化学吸附,O 2 -TPD和CO-TPR。CO解吸和O 2解离的能力越强,CO 2越容易发现形成Pd较小的Pd较高的活性。我们相信,我们的发现代表了开发高效催化剂的潜在策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号