当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Formal Hydroamidation of Si-Substituted Arylacetylenes with DIBAL-H and Isocyanates: Synthesis of (E)- and (Z)-α-Silyl-α,β-unsaturated Amides.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.joc.0c01903 Hanseul Lee 1 , Soohong Cho 1 , Yunmi Lee 1 , Byunghyuck Jung 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.joc.0c01903 Hanseul Lee 1 , Soohong Cho 1 , Yunmi Lee 1 , Byunghyuck Jung 2
Affiliation
|
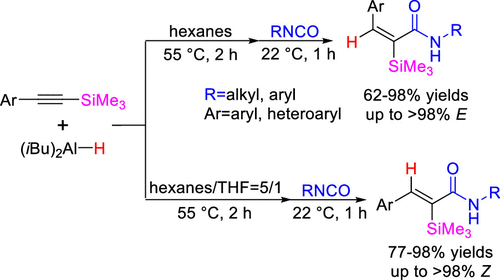
An efficient and stereoselective method for the synthesis of (E)- and (Z)-α-silyl-α,β-unsaturated amides and its synthetic applications are presented herein. The solvent-controlled hydroaluminations of Si-substituted alkynes with DIBAL-H generate diastereomerically enriched alkenylaluminum reagents that are directly reacted with isocyanates at ambient temperature to afford α-silyl-α,β-unsaturated amides in high yields with retained stereoselectivity. In particular, this process enables the synthesis of a broad range of (E)-α-silyl-α,β-unsaturated amides, which are the less studied isomers. The synthetic utility of this method is highlighted by its short reaction time, ease of purification, easily accessible substrates and reagents, gram-scale synthesis, and the further transformations of C–Si bonds into C–H, C–X, and C–C bonds.
中文翻译:

用DIBAL-H和异氰酸酯对Si取代的芳基乙炔进行立体选择性形式的氢酰胺化:(E)-和(Z)-α-甲硅烷基-α,β-不饱和酰胺的合成。
本文提出了一种合成(E)-和(Z)-α-甲硅烷基-α,β-不饱和酰胺的有效且立体选择性的方法及其合成应用。用DIBAL-H进行的溶剂控制的Si取代炔烃的水铝化反应可生成富含非对映异构体的烯基铝试剂,这些试剂可在环境温度下与异氰酸酯直接反应,以高产率得到α-甲硅烷基-α,β-不饱和酰胺,并保留了立体选择性。尤其是,此过程可以合成多种(E)-α-甲硅烷基-α,β-不饱和酰胺,是研究较少的异构体。该方法的合成实用性因其反应时间短,易于纯化,容易获得的底物和试剂,克级合成以及C–Si键进一步转化为C–H,C–X和C–的特点而突出。 C键。
更新日期:2020-10-02
中文翻译:

用DIBAL-H和异氰酸酯对Si取代的芳基乙炔进行立体选择性形式的氢酰胺化:(E)-和(Z)-α-甲硅烷基-α,β-不饱和酰胺的合成。
本文提出了一种合成(E)-和(Z)-α-甲硅烷基-α,β-不饱和酰胺的有效且立体选择性的方法及其合成应用。用DIBAL-H进行的溶剂控制的Si取代炔烃的水铝化反应可生成富含非对映异构体的烯基铝试剂,这些试剂可在环境温度下与异氰酸酯直接反应,以高产率得到α-甲硅烷基-α,β-不饱和酰胺,并保留了立体选择性。尤其是,此过程可以合成多种(E)-α-甲硅烷基-α,β-不饱和酰胺,是研究较少的异构体。该方法的合成实用性因其反应时间短,易于纯化,容易获得的底物和试剂,克级合成以及C–Si键进一步转化为C–H,C–X和C–的特点而突出。 C键。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号