当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Synthesis of Bicyclo[3.2.1]octenes and Cyclohexenes via Catalytic Annulations of Nazarov Reagent and Vinyl 1,2-Diketones.
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.orglett.0c02763 Wenjun Liu 1 , Shengtong Niu 1 , Zhifei Zhao 1 , Shuang Yang 1 , Jinggong Liu 2 , Yongjin Li 2 , Xinqiang Fang 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-10 , DOI: 10.1021/acs.orglett.0c02763 Wenjun Liu 1 , Shengtong Niu 1 , Zhifei Zhao 1 , Shuang Yang 1 , Jinggong Liu 2 , Yongjin Li 2 , Xinqiang Fang 1
Affiliation
|
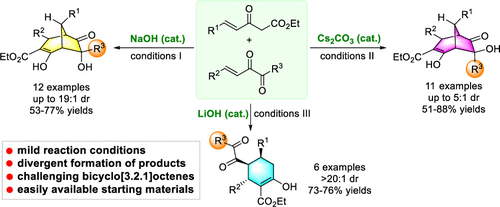
Bicyclo[3.2.1]octanes and related structures are unique units that widely exist in natural products, but the rapid and stereoselective construction of this skeleton is a challenging issue. We report the stereodivergent synthesis of bicyclo[3.2.1]octenes using Nazarov reagents and alkenyl 1,2-diketones with Brønsted base catalysis under mild conditions. Both stereoisomers of the bridged products can be obtained by tuning the reaction conditions, and cyclohexene product can also be selectively formed.
中文翻译:

通过纳扎罗夫试剂和1,2-乙烯基二酮的催化环化反应合成双环[3.2.1]辛烯和环己烯。
双环[3.2.1]辛烷和相关结构是在天然产物中广泛存在的独特单元,但是该骨架的快速和立体选择性构造是一个具有挑战性的问题。我们报告在温和的条件下使用Nazarov试剂和烯基1,2-二酮与Brønsted碱催化的立体发散合成双环[3.2.1]辛烯。桥连产物的两种立体异构体可以通过调节反应条件来获得,并且环己烯产物也可以选择性地形成。
更新日期:2020-10-02
中文翻译:

通过纳扎罗夫试剂和1,2-乙烯基二酮的催化环化反应合成双环[3.2.1]辛烯和环己烯。
双环[3.2.1]辛烷和相关结构是在天然产物中广泛存在的独特单元,但是该骨架的快速和立体选择性构造是一个具有挑战性的问题。我们报告在温和的条件下使用Nazarov试剂和烯基1,2-二酮与Brønsted碱催化的立体发散合成双环[3.2.1]辛烯。桥连产物的两种立体异构体可以通过调节反应条件来获得,并且环己烯产物也可以选择性地形成。













































 京公网安备 11010802027423号
京公网安备 11010802027423号