当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Capturing the active sites of multimetallic (oxy)hydroxides for the oxygen evolution reaction
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-09-09 , DOI: 10.1039/d0ee01609h
Xin Bo 1, 2, 3, 4 , Rosalie K Hocking 4, 5, 6, 7, 8 , Si Zhou 4, 9, 10, 11, 12 , Yibing Li 1, 2, 3, 4 , Xianjue Chen 1, 2, 3, 4 , Jincheng Zhuang 13, 14, 15, 16 , Yi Du 4, 9, 10, 11, 12 , Chuan Zhao 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-09-09 , DOI: 10.1039/d0ee01609h
Xin Bo 1, 2, 3, 4 , Rosalie K Hocking 4, 5, 6, 7, 8 , Si Zhou 4, 9, 10, 11, 12 , Yibing Li 1, 2, 3, 4 , Xianjue Chen 1, 2, 3, 4 , Jincheng Zhuang 13, 14, 15, 16 , Yi Du 4, 9, 10, 11, 12 , Chuan Zhao 1, 2, 3, 4
Affiliation
|
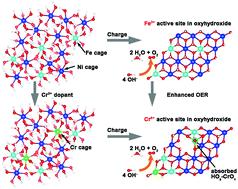
Efficient generation of H2 via water-splitting is an underpinning technology for realizing the hydrogen economy. However, the sluggish anodic oxygen evolution reaction (OER) requires a large energy input. Low-cost, transition metals such as NiFe oxides/hydroxides have been regarded as one of the most efficient catalysts for the OER in alkaline media, although the detailed mechanisms remain debated due to the lack of direct evidence for the proposed active sites during the catalytic processes. Herein, we show a NiFe (oxy)hydroxide catalyst doped with a third metal Cr prepared by facile electrodeposition to achieve further enhanced activity for the OER. Operando Raman and X-ray absorption spectroscopy (XAS) characterisation were employed to detect the formation of active intermediates and M–O bonds on active sites during the OER process. For the host NiFe (oxy)hydroxide catalyst, the shorter Fe–O in the Fe-substituted-β-NiOOH intermediate is observed as active sites for the OER. A Cr, Fe-substituted-β-NiOOH intermediate is detected in the enhanced NiFeCr (oxy)hydroxide catalyst where Cr is oxidized into the 6+ valence state with optimal Cr–O bonds, adding new active sites to boost the OER. Density functional theory (DFT) calculations support the operando spectroscopic observations and reveal the lower overpotential at the Cr6+ sites in the NiFeCr oxyhydroxide intermediate than the Fe3+ sites in the NiFe oxyhydroxide intermediate. This study demonstrates a strategy for designing highly active OER catalysts by introducing high valence metals into oxides/hydroxides to further enhance the kinetics of water oxidation.
中文翻译:

捕获多金属(氧)氢氧化物的活性位以进行放氧反应
通过水分解有效产生H 2 是实现氢经济的基础技术。但是,缓慢的阳极氧气析出反应(OER)需要大量的能量输入。低成本的过渡金属(例如NiFe氧化物/氢氧化物)被认为是碱性介质中OER的最有效催化剂之一,尽管由于缺乏直接证据表明催化过程中拟议的活性位点,详细的机理仍存在争议流程。在本文中,我们显示了掺有通过便捷的电沉积制备的第三金属Cr的NiFe(羟基)氢氧化物催化剂,以进一步提高OER的活性。Operando拉曼光谱和X射线吸收光谱(XAS)表征用于检测OER过程中活性中间体和活性位点上的M-O键的形成。对于主体NiFe(羟基)氢氧化物催化剂,观察到Fe取代的β-NiOOH中间体中较短的Fe–O是OER的活性位点。在增强的NiFeCr(羟基)氢氧化物催化剂中检测到Cr,Fe取代的β-NiOOH中间体,其中Cr被氧化为具有最佳Cr–O键的6+价态,从而增加了新的活性位点以提高OER。密度泛函理论(DFT)计算支持操作光谱学观察,并揭示了NiFeCr羟基氧化中间产物中Cr 6+位点的过电势低于Fe 3+NiFe羟基氢氧化物中间体中的位点。这项研究表明了通过将高价金属引入氧化物/氢氧化物以进一步增强水氧化动力学来设计高活性OER催化剂的策略。
更新日期:2020-11-03
中文翻译:

捕获多金属(氧)氢氧化物的活性位以进行放氧反应
通过水分解有效产生H 2 是实现氢经济的基础技术。但是,缓慢的阳极氧气析出反应(OER)需要大量的能量输入。低成本的过渡金属(例如NiFe氧化物/氢氧化物)被认为是碱性介质中OER的最有效催化剂之一,尽管由于缺乏直接证据表明催化过程中拟议的活性位点,详细的机理仍存在争议流程。在本文中,我们显示了掺有通过便捷的电沉积制备的第三金属Cr的NiFe(羟基)氢氧化物催化剂,以进一步提高OER的活性。Operando拉曼光谱和X射线吸收光谱(XAS)表征用于检测OER过程中活性中间体和活性位点上的M-O键的形成。对于主体NiFe(羟基)氢氧化物催化剂,观察到Fe取代的β-NiOOH中间体中较短的Fe–O是OER的活性位点。在增强的NiFeCr(羟基)氢氧化物催化剂中检测到Cr,Fe取代的β-NiOOH中间体,其中Cr被氧化为具有最佳Cr–O键的6+价态,从而增加了新的活性位点以提高OER。密度泛函理论(DFT)计算支持操作光谱学观察,并揭示了NiFeCr羟基氧化中间产物中Cr 6+位点的过电势低于Fe 3+NiFe羟基氢氧化物中间体中的位点。这项研究表明了通过将高价金属引入氧化物/氢氧化物以进一步增强水氧化动力学来设计高活性OER催化剂的策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号