当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption and Oxidation Dynamics of Disperse Orange 3 on a Polycrystalline Pt Electrode Studied by In Situ Second Harmonic Generation
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.jpcc.0c07393 Ruipeng Bai 1, 2 , Man Xue 1, 2 , Yuan Lin 2, 3 , Rui Wen 2, 4 , Yuan Guo 1, 2 , Zhen Zhang 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-09-08 , DOI: 10.1021/acs.jpcc.0c07393 Ruipeng Bai 1, 2 , Man Xue 1, 2 , Yuan Lin 2, 3 , Rui Wen 2, 4 , Yuan Guo 1, 2 , Zhen Zhang 1, 2
Affiliation
|
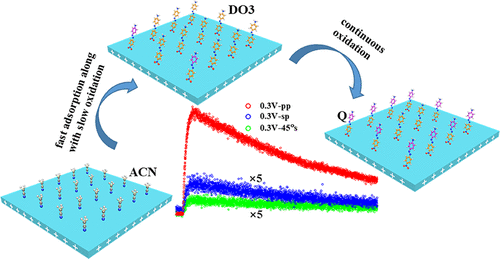
Adsorption mechanisms and electrochemical reaction mechanisms at the molecular level are fundamental issues in electrochemistry. Here, we used in situ second harmonic generation (SHG) combined with in situ UV–vis spectra and potential step techniques to investigate the dynamics of the adsorption and oxidation of 4-amino-4′-nitroazobenzene (disperse orange 3, DO3) molecules on a polycrystalline Pt electrode. Time-dependent SHG spectra with different polarization combinations under certain potential steps confirmed that the SHG intensity change in the DO3 molecules resulted from the number density change rather than the orientation angle change. A double potential step experiment and UV–vis spectra verified that the number density change arose from the cooperative effects of adsorption and oxidation of DO3 at the interfaces. Under an applied potential, the adsorption rate of DO3 was greater than the electrooxidation rate in the first hundred seconds until full coverage was achieved and the maximum SHG intensity was reached. Subsequently, the oxidation of DO3 dominated until equilibrium was established, while the SHG intensity stabilized. In addition, the time-dependent SHG spectra showed that the rate constants of the adsorption process and the oxidation reaction were strongly dependent on the potential. This work provides in-depth insights into the adsorption and electrochemical reaction at electrode/solution interfaces.
中文翻译:

原位二次谐波研究多晶铂电极上分散橙3的吸附和氧化动力学
在分子水平上的吸附机理和电化学反应机理是电化学中的基本问题。在这里,我们使用原位二次谐波(SHG)结合原位UV-vis光谱和电位阶跃技术研究了4-氨基-4'-硝基偶氮苯(分散橙3,DO3)分子的吸附和氧化动力学。在多晶铂电极上。在某些可能的步长下具有不同极化组合的随时间变化的SHG光谱证实,DO3分子中的SHG强度变化是由数密度变化而不是取向角变化引起的。双重电位步骤实验和UV-vis光谱证明,密度的变化是由于界面处DO3的吸附和氧化的协同作用引起的。在施加电势的情况下,直到获得完全覆盖并达到最大SHG强度之前,DO3的吸附速率大于前100秒的电氧化速率。随后,DO3的氧化占主导地位,直到建立平衡,而SHG强度稳定。此外,随时间变化的SHG光谱表明,吸附过程和氧化反应的速率常数强烈依赖于电势。这项工作提供了对电极/溶液界面处的吸附和电化学反应的深入了解。DO3的氧化占主导地位,直到建立平衡,而SHG强度稳定。此外,随时间变化的SHG光谱表明,吸附过程和氧化反应的速率常数强烈依赖于电势。这项工作提供了对电极/溶液界面处的吸附和电化学反应的深入了解。DO3的氧化占主导地位,直到建立平衡,而SHG强度稳定。此外,随时间变化的SHG光谱表明,吸附过程和氧化反应的速率常数强烈依赖于电势。这项工作提供了对电极/溶液界面处的吸附和电化学反应的深入了解。
更新日期:2020-10-02
中文翻译:

原位二次谐波研究多晶铂电极上分散橙3的吸附和氧化动力学
在分子水平上的吸附机理和电化学反应机理是电化学中的基本问题。在这里,我们使用原位二次谐波(SHG)结合原位UV-vis光谱和电位阶跃技术研究了4-氨基-4'-硝基偶氮苯(分散橙3,DO3)分子的吸附和氧化动力学。在多晶铂电极上。在某些可能的步长下具有不同极化组合的随时间变化的SHG光谱证实,DO3分子中的SHG强度变化是由数密度变化而不是取向角变化引起的。双重电位步骤实验和UV-vis光谱证明,密度的变化是由于界面处DO3的吸附和氧化的协同作用引起的。在施加电势的情况下,直到获得完全覆盖并达到最大SHG强度之前,DO3的吸附速率大于前100秒的电氧化速率。随后,DO3的氧化占主导地位,直到建立平衡,而SHG强度稳定。此外,随时间变化的SHG光谱表明,吸附过程和氧化反应的速率常数强烈依赖于电势。这项工作提供了对电极/溶液界面处的吸附和电化学反应的深入了解。DO3的氧化占主导地位,直到建立平衡,而SHG强度稳定。此外,随时间变化的SHG光谱表明,吸附过程和氧化反应的速率常数强烈依赖于电势。这项工作提供了对电极/溶液界面处的吸附和电化学反应的深入了解。DO3的氧化占主导地位,直到建立平衡,而SHG强度稳定。此外,随时间变化的SHG光谱表明,吸附过程和氧化反应的速率常数强烈依赖于电势。这项工作提供了对电极/溶液界面处的吸附和电化学反应的深入了解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号