当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselectivity Control in Pd-Catalyzed Telomerization of Isoprene Enabled by Solvent and Ligand Selection
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-09-09 , DOI: 10.1021/acscatal.0c02911 Jordi Colavida 1 , José A. Lleberia 2 , Antoni Salom-Català 2 , Aitor Gual 1 , Ana Collado 3 , Ennio Zangrando 4 , Josep M. Ricart 2 , Cyril Godard 2 , Carmen Claver 1, 2 , Jorge J. Carbó 2 , Sergio Castillon 1, 5
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-09-09 , DOI: 10.1021/acscatal.0c02911 Jordi Colavida 1 , José A. Lleberia 2 , Antoni Salom-Català 2 , Aitor Gual 1 , Ana Collado 3 , Ennio Zangrando 4 , Josep M. Ricart 2 , Cyril Godard 2 , Carmen Claver 1, 2 , Jorge J. Carbó 2 , Sergio Castillon 1, 5
Affiliation
|
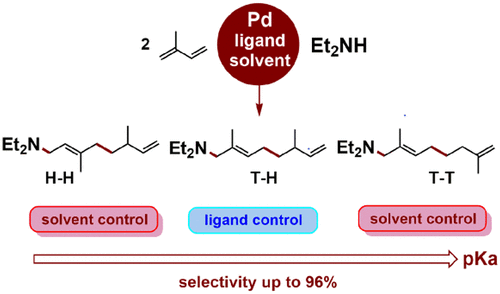
Controlling the selectivity in palladium-catalyzed telomerization of nonsymmetric dienes represents a formidable challenge since up to 12 isomers can be obtained, and a general method for selective synthesis is still lacking. We select isoprene (2-methylbutadiene) as a representative and relevant example of a nonsymmetric diene. A combined experimental–computational study on a large set of phosphine-modified palladium catalysts and reaction conditions aiming to understand the factors governing the selectivity shows that it can be controlled by selecting the protic solvent pKa and by the ligand. Atomistic and kinetic simulations reveal that the solvent switches the selectivity-determining step as a function of pKa from C–C oxidative coupling at low pKa values (preference for telomer head-to-head) to protonation at high pKa values (preference for telomer tail-to-tail). The selectivity toward tail-to-head telomer can be directed in moderately acidic solvents by selection of the appropriate ligand, which exerts steric control of the protonation step. Thus, using Et2NH as a nucleophile, it was possible to synthesize 3 of the 4 main isomers in very high yields and selectivities and to provide a complete mechanistic picture of Pd-catalyzed telomerization of nonsymmetric dienes.
中文翻译:

通过溶剂和配体选择实现的Pd催化异戊二烯端粒化中的区域选择性控制
由于可获得多达12种异构体,控制钯催化的非对称二烯端粒化反应中的选择性是一个艰巨的挑战,并且仍然缺乏通用的选择性合成方法。我们选择异戊二烯(2-甲基丁二烯)作为非对称二烯的代表和相关实例。旨在了解选择性决定因素的大批膦改性钯催化剂和反应条件的组合实验计算研究表明,可以通过选择质子溶剂p K a和配体来控制它。原子和动力学模拟表明,溶剂在低p时根据C–C氧化偶合切换选择性确定步骤与p K a的关系在高p K a值(端粒尾对尾的偏好)下,质子化的K a值(端粒头对端的偏好)。通过选择合适的配体,可以在中等酸性溶剂中控制对尾对头端粒的选择性,从而对质子化步骤进行空间控制。因此,使用Et 2 NH作为亲核试剂,可以以很高的产率和选择性合成4种主要异构体中的3种,并提供了Pd催化的非对称二烯端粒化的完整机理图。
更新日期:2020-10-02
中文翻译:

通过溶剂和配体选择实现的Pd催化异戊二烯端粒化中的区域选择性控制
由于可获得多达12种异构体,控制钯催化的非对称二烯端粒化反应中的选择性是一个艰巨的挑战,并且仍然缺乏通用的选择性合成方法。我们选择异戊二烯(2-甲基丁二烯)作为非对称二烯的代表和相关实例。旨在了解选择性决定因素的大批膦改性钯催化剂和反应条件的组合实验计算研究表明,可以通过选择质子溶剂p K a和配体来控制它。原子和动力学模拟表明,溶剂在低p时根据C–C氧化偶合切换选择性确定步骤与p K a的关系在高p K a值(端粒尾对尾的偏好)下,质子化的K a值(端粒头对端的偏好)。通过选择合适的配体,可以在中等酸性溶剂中控制对尾对头端粒的选择性,从而对质子化步骤进行空间控制。因此,使用Et 2 NH作为亲核试剂,可以以很高的产率和选择性合成4种主要异构体中的3种,并提供了Pd催化的非对称二烯端粒化的完整机理图。

































 京公网安备 11010802027423号
京公网安备 11010802027423号