当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Liangshanone.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-09 , DOI: 10.1002/anie.202011923 Hong-Xiu Huang 1 , Fen Mi 1 , Chunxin Li 1 , Huan He 1 , Feng-Peng Wang 1 , Xiao-Yu Liu 1 , Yong Qin 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-09 , DOI: 10.1002/anie.202011923 Hong-Xiu Huang 1 , Fen Mi 1 , Chunxin Li 1 , Huan He 1 , Feng-Peng Wang 1 , Xiao-Yu Liu 1 , Yong Qin 1
Affiliation
|
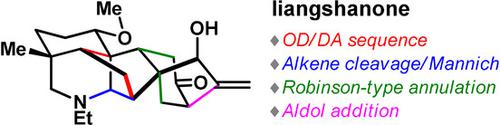
The first total synthesis of liangshanone, a hexacyclic ent‐kaurane diterpenoid alkaloid, has been completed. Its intricate cagelike framework was assembled through several key transformations, including an oxidative dearomatization/Diels–Alder (OD/DA) cycloaddition sequence, a tandem alkene cleavage/Mannich cyclization, a Robinson‐type annulation, and an intramolecular aldol reaction. Notably, an organocatalytic enantioselective α‐hydroxymethylation process allowed the preparation of an enantiomerically enriched tricyclic intermediate that should enable asymmetric access to the target natural product.
中文翻译:

梁山酮的全合成。
liangshanone,六环的第一全合成ENT -kaurane二萜生物碱,已经完成。它复杂的笼状框架是通过几个关键的转换组装而成的,包括氧化脱芳香化/ Diels-Alder(OD / DA)环加成序列,串联烯烃裂解/ Mannich环化,Robinson型环化和分子内羟醛反应。值得注意的是,有机催化对映选择性α-羟甲基化过程允许制备对映体富集的三环中间体,该中间体应能够不对称地接近目标天然产物。
更新日期:2020-09-09
中文翻译:

梁山酮的全合成。
liangshanone,六环的第一全合成ENT -kaurane二萜生物碱,已经完成。它复杂的笼状框架是通过几个关键的转换组装而成的,包括氧化脱芳香化/ Diels-Alder(OD / DA)环加成序列,串联烯烃裂解/ Mannich环化,Robinson型环化和分子内羟醛反应。值得注意的是,有机催化对映选择性α-羟甲基化过程允许制备对映体富集的三环中间体,该中间体应能够不对称地接近目标天然产物。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号