当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-bromomethyl-2,3-dihydrobenzofurans from 2-allylphenols enabled by organocatalytic activation of N-bromosuccinimide
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-09-07 , DOI: 10.1039/d0nj03432k Carolina G. Furst 1, 2, 3, 4 , Paulo H. P. Cota 1, 2, 3, 4 , Taciano A. dos Santos Wanderley 1, 2, 3, 4 , Eduardo E. Alberto 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-09-07 , DOI: 10.1039/d0nj03432k Carolina G. Furst 1, 2, 3, 4 , Paulo H. P. Cota 1, 2, 3, 4 , Taciano A. dos Santos Wanderley 1, 2, 3, 4 , Eduardo E. Alberto 1, 2, 3, 4
Affiliation
|
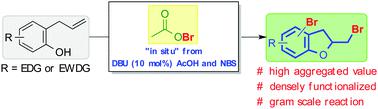
2-Bromomethyl-2,3-dihydrobenzofurans are valuable and highly functionalized compounds that can be obtained by an intramolecular reaction between 2-allylphenols and a bromenium ion source (Br+). Due to the ineffectiveness of the safe and easy-to-handle brominating agent N-bromosuccinimide (NBS) to deliver the desired products, a catalytic process using a mixture of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and acetic acid was conceived. We hypothesized that this catalytic system delivers in situ acetyl hypobromite (AcOBr) as the active brominating agent, enabling the conversion of a range of 2-allylphenols with diverse electron densities to the products. The protocol was robust enough to permit the reaction to be scaled up to 10 mmols of starting material. Besides, the functional group interconversion with a 2-bromomethyl-2,3-dihydrobenzofuran derivative was successfully demonstrated.
中文翻译:

N-溴代琥珀酰亚胺的有机催化活化由2-烯丙基苯酚合成2-溴甲基-2,3-二氢苯并呋喃
2-溴甲基-2,3-二氢苯并呋喃是有价值的且高度官能化的化合物,可以通过2-烯丙基苯酚和溴离子源(Br +)之间的分子内反应获得。由于安全易处理的溴化剂N-溴代琥珀酰亚胺(NBS)不能有效地递送所需产物,因此需要使用1,8-二氮杂双环[5.4.0]十一碳-7-烯(设想了DBU)和乙酸。我们假设该催化系统原位输送乙酰基次溴酸盐(AcOBr)作为活性溴化剂,可将一系列具有不同电子密度的2-烯丙基苯酚转化为产物。该方案足够鲁棒,可以使反应规模扩大到10 mmols的起始原料。此外,成功地证明了与2-溴甲基-2,3-二氢苯并呋喃衍生物的官能团互变。
更新日期:2020-09-21
中文翻译:

N-溴代琥珀酰亚胺的有机催化活化由2-烯丙基苯酚合成2-溴甲基-2,3-二氢苯并呋喃
2-溴甲基-2,3-二氢苯并呋喃是有价值的且高度官能化的化合物,可以通过2-烯丙基苯酚和溴离子源(Br +)之间的分子内反应获得。由于安全易处理的溴化剂N-溴代琥珀酰亚胺(NBS)不能有效地递送所需产物,因此需要使用1,8-二氮杂双环[5.4.0]十一碳-7-烯(设想了DBU)和乙酸。我们假设该催化系统原位输送乙酰基次溴酸盐(AcOBr)作为活性溴化剂,可将一系列具有不同电子密度的2-烯丙基苯酚转化为产物。该方案足够鲁棒,可以使反应规模扩大到10 mmols的起始原料。此外,成功地证明了与2-溴甲基-2,3-二氢苯并呋喃衍生物的官能团互变。






























 京公网安备 11010802027423号
京公网安备 11010802027423号