当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interfacial structure of water as a new descriptor of hydrogen evolution reaction.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-06 , DOI: 10.1002/anie.202007567 Lin-Fan Shen 1 , Bang-An Lu 1 , Yu-Yang Li 1 , Jia Liu 1 , Zhi-Chao Huang-Fu 1 , Hao Peng 1 , Jin-Yu Ye 1 , Xi-Ming Qu 1 , Jun-Ming Zhang 1 , Guang Li 1 , Wen-Bin Cai 2 , Yan-Xia Jiang 1 , Shi-Gang Sun 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-06 , DOI: 10.1002/anie.202007567 Lin-Fan Shen 1 , Bang-An Lu 1 , Yu-Yang Li 1 , Jia Liu 1 , Zhi-Chao Huang-Fu 1 , Hao Peng 1 , Jin-Yu Ye 1 , Xi-Ming Qu 1 , Jun-Ming Zhang 1 , Guang Li 1 , Wen-Bin Cai 2 , Yan-Xia Jiang 1 , Shi-Gang Sun 1
Affiliation
|
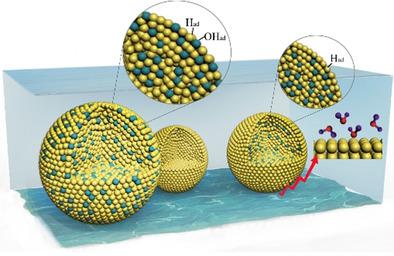
Driven by the persisting poor understanding of the sluggish kinetics of the hydrogen evolution reaction (HER) on Pt in alkaline media, a direct correlation of the interfacial water structure and activity is still yet to be established. Herein, using Pt and Pt–Ni nanoparticles we first demonstrate a strong dependence of the proton donor structure on the HER activity and pH. The structure of the first layer changes from the proton acceptors to the donors with increasing pH. In the base, the reactivity of the interfacial water varied its structure, and the activation energies of water dissociation increased in the sequence: the dangling O−H bonds < the trihedrally coordinated water < the tetrahedrally coordinated water. Moreover, optimizing the adsorption of H and OH intermediates can re‐orientate the interfacial water molecules with their H atoms pointing towards the electrode surface, thereby enhancing the kinetics of HER. Our results clarified the dynamic role of the water structure at the electrode–electrolyte interface during HER and the design of highly efficient HER catalysts.
中文翻译:

水的界面结构是析氢反应的新描述。
由于人们对碱性介质中Pt上的析氢反应(HER)的缓慢动力学的认识不强,因此尚需建立界面水结构与活性之间的直接关系。在此,我们首先使用Pt和Pt-Ni纳米粒子证明了质子供体结构对HER活性和pH的强烈依赖性。随着pH值的增加,第一层的结构从质子受体改变为供体。在此基础上,界面水的反应性改变了其结构,水解离的活化能依次增加:悬空的OH键<三面体配位水<四面体配位水。此外,优化H和OH中间体的吸附可重新定向界面水分子,使其H原子指向电极表面,从而增强HER的动力学。我们的结果阐明了在HER期间电极与电解质界面处水结构的动态作用以及高效HER催化剂的设计。
更新日期:2020-09-06
中文翻译:

水的界面结构是析氢反应的新描述。
由于人们对碱性介质中Pt上的析氢反应(HER)的缓慢动力学的认识不强,因此尚需建立界面水结构与活性之间的直接关系。在此,我们首先使用Pt和Pt-Ni纳米粒子证明了质子供体结构对HER活性和pH的强烈依赖性。随着pH值的增加,第一层的结构从质子受体改变为供体。在此基础上,界面水的反应性改变了其结构,水解离的活化能依次增加:悬空的OH键<三面体配位水<四面体配位水。此外,优化H和OH中间体的吸附可重新定向界面水分子,使其H原子指向电极表面,从而增强HER的动力学。我们的结果阐明了在HER期间电极与电解质界面处水结构的动态作用以及高效HER催化剂的设计。































 京公网安备 11010802027423号
京公网安备 11010802027423号