当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular-Weight Growth in Ozone-Initiated Low-Temperature Oxidation of Methyl Crotonate.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-09-05 , DOI: 10.1021/acs.jpca.0c05684 X He 1 , N Hansen 2 , K Moshammer 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-09-05 , DOI: 10.1021/acs.jpca.0c05684 X He 1 , N Hansen 2 , K Moshammer 1
Affiliation
|
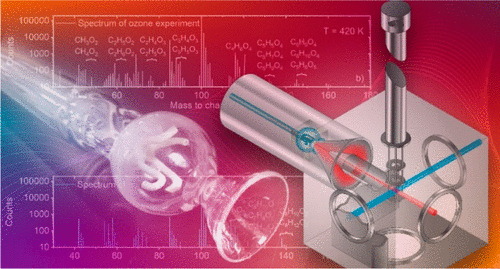
We report experiments of ozone-initiated low-temperature oxidation of methyl crotonate (MC, CH3—CH═CH—C(O)OCH3) from 420 to 660 K in a near-atmospheric-pressure jet-stirred reactor using photoionization molecular-beam mass spectrometry as a sampling technique. In this temperature regime, no typical low-temperature combustion (LTC) reactions have been observed for MC when oxygen (O2) is used as the oxidizer. Upon ozone addition, significant oxidation of methyl crotonate is found. On the basis of experimentally observed energy-dependent mass peaks in combination with temperature-dependent mole fraction profiles and photoionization efficiency curves, we provide new insights into the methyl crotonate ozonolysis reaction network. The observed MC + O3 products, C5H8O5, are found to be related to the keto-hydroperoxides resulting from the isomerization of the primary ozonide. Evidence is also provided that molecular growth mainly results from cycloaddition reactions of the Criegee intermediate into aldehydes and alkenes as well as addition reactions of the Criegee intermediates to the double bond of methyl crotonate and sequential decomposition into ketones. Furthermore, species that contribute in large amounts to the low-temperature oxidation of methyl crotonate, like H2O2, CH3OOH, CH3OH, and HC(O)OH, are identified, and their mole fractions are reported. Additionally, preliminary modeling is performed which qualitatively captures the observed NTC behavior and reveals future research opportunities.
中文翻译:

臭氧引发的巴豆酸甲酯低温氧化中的分子重量增长。
我们报告了在近大气压射流搅拌反应器中使用光电离分子对臭氧引发的巴豆酸甲酯(MC,CH 3 -CH═CH-C(O)OCH 3)从420至660 K进行低温氧化的实验束质谱法作为一种采样技术。在此温度范围内,当使用氧气(O 2)作为氧化剂时,没有观察到MC发生典型的低温燃烧(LTC)反应。加入臭氧后,发现巴豆酸甲酯发生了明显的氧化。基于实验观察到的能量相关的质量峰,以及温度相关的摩尔分数分布和光电离效率曲线,我们为巴豆酸甲酯臭氧分解反应网络提供了新见解。观察到的MC + O 3发现产物C 5 H 8 O 5与由初级臭氧化物的异构化产生的酮氢过氧化物有关。还提供了分子生长的主要原因是Criegee中间体向醛和烯烃的环加成反应以及Criegee中间体与巴豆酸甲酯双键的加成反应和依次分解为酮的结果。此外,H 2 O 2,CH 3 OOH,CH 3等对巴豆酸甲酯的低温氧化有很大贡献的物质确定了OH和HC(O)OH,并报告了其摩尔分数。此外,执行初步建模可以定性地捕获观察到的NTC行为并揭示未来的研究机会。
更新日期:2020-10-02
中文翻译:

臭氧引发的巴豆酸甲酯低温氧化中的分子重量增长。
我们报告了在近大气压射流搅拌反应器中使用光电离分子对臭氧引发的巴豆酸甲酯(MC,CH 3 -CH═CH-C(O)OCH 3)从420至660 K进行低温氧化的实验束质谱法作为一种采样技术。在此温度范围内,当使用氧气(O 2)作为氧化剂时,没有观察到MC发生典型的低温燃烧(LTC)反应。加入臭氧后,发现巴豆酸甲酯发生了明显的氧化。基于实验观察到的能量相关的质量峰,以及温度相关的摩尔分数分布和光电离效率曲线,我们为巴豆酸甲酯臭氧分解反应网络提供了新见解。观察到的MC + O 3发现产物C 5 H 8 O 5与由初级臭氧化物的异构化产生的酮氢过氧化物有关。还提供了分子生长的主要原因是Criegee中间体向醛和烯烃的环加成反应以及Criegee中间体与巴豆酸甲酯双键的加成反应和依次分解为酮的结果。此外,H 2 O 2,CH 3 OOH,CH 3等对巴豆酸甲酯的低温氧化有很大贡献的物质确定了OH和HC(O)OH,并报告了其摩尔分数。此外,执行初步建模可以定性地捕获观察到的NTC行为并揭示未来的研究机会。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号