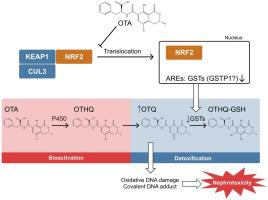
赭曲霉毒素 A (OTA) 是食品中最丰富的霉菌毒素污染物之一,具有致癌、肾毒性、致畸和免疫毒性特性。具体来说,一个主要问题是严重的肾毒性,其特征是近端肾小管上皮细胞变性和间质纤维化。然而,由于缺乏充分模拟人类肾功能的体外模型,OTA 毒性的机制以及导致其对人类毒性的遗传风险因素一直难以捉摸。本研究试图利用 3D 人肾近曲小管微生理系统(肾MPS)。我们证明肾脏 MPS 培养物中 OTA 的 LC 50值 (0.375–1.21 μM) 与尿液中 OTA 的临床相关毒性浓度一致。令人惊讶的是,尽管 LIVE/DEAD 染色观察到显着的毒性,但 OTA 暴露后肾脏 MPS 流出物中的肾损伤生物标志物并未明显增强。相反,这些生物标志物以 OTA 浓度依赖性方式下降。此外,1-氨基苯并三唑 (ABT) 和 6-(7-硝基-2,1,3-苯并恶二唑-4-基硫基)己醇 (NBDHEX)、P450 和谷胱甘肽 S-转移酶 (GST) 的泛抑制剂的作用分别对 OTA 诱导的肾脏 MPS 毒性进行了检查。这些研究表明,NBDHEX (3 μM) 处理显着增强了 OTA 诱导的毒性,而 ABT (1 mM) 处理则降低了 OTA 诱导的毒性,表明 GST 和 P450 酶分别在 OTA 解毒和生物激活中发挥作用。使用不同浓度 OTA 处理的肾脏 MPS 进行 RNA 测序分析转录变化,结果显示 OTA 处理后,多个核因子(红细胞衍生 2)样 2 (NRF2) 调节的基因(包括 GST)下调。GST 的转录抑制可能通过减弱谷胱甘肽结合/解毒而在 OTA 毒性中发挥关键作用。连续的分子事件可以解释与 OTA 相关的毒性机制。此外,在存在和不存在丙磺舒 (1 mM) 的情况下使用肾脏 MPS 进行的 OTA 转运研究表明,有机阴离子膜转运蛋白在 OTA 的肾脏特异性处置中发挥着作用。我们的研究结果使人们更清楚地了解 OTA 引起的肾损伤的机制,这可能支持风险评估、监管机构关于允许暴露水平的政策的变化,以及确定遗传因素在 OTA 肾毒性风险人群中的作用。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Microphysiological system modeling of ochratoxin A-associated nephrotoxicity.
Ochratoxin A (OTA) is one of the most abundant mycotoxin contaminants in food stuffs and possesses carcinogenic, nephrotoxic, teratogenic, and immunotoxic properties. Specifically, a major concern is severe nephrotoxicity, which is characterized by degeneration of epithelial cells of the proximal tubules and interstitial fibrosis. However, the mechanism of OTA toxicity, as well as the genetic risk factors contributing to its toxicity in humans has been elusive due to the lack of adequate models that fully recapitulate human kidney function in vitro. The present study attempts to evaluate dose-response relationships, identify the contribution of active transport proteins that govern the renal disposition of OTA, and determine the role of metabolism in the bioactivation and detoxification of OTA using a 3D human kidney proximal tubule microphysiological system (kidney MPS). We demonstrated that LC50 values of OTA in kidney MPS culture (0.375–1.21 μM) were in agreement with clinically relevant toxic concentrations of OTA in urine. Surprisingly, no enhancement of kidney injury biomarkers was evident in the effluent of the kidney MPS after OTA exposure despite significant toxicity observed by LIVE/DEAD staining. Instead, these biomarkers decreased in an OTA concentration-dependent manner. Furthermore, the effect of 1-aminobenzotriazole (ABT) and 6-(7-Nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol (NBDHEX), pan-inhibitors of P450 and glutathione S-transferase (GST) enzymes, respectively, on OTA-induced toxicity in kidney MPS was examined. These studies revealed significant enhancement of OTA-induced toxicity by NBDHEX (3 μM) treatment, whereas ABT (1 mM) treatment decreased OTA-induced toxicity, suggesting roles for GSTs and P450 enzymes in the detoxification and bioactivation of OTA, respectively. Analysis of transcriptional changes using RNA-sequencing of kidney MPS treated with different concentrations of OTA revealed downregulation of several nuclear factor (erythroid derived-2)-like 2 (NRF2)-regulated genes by OTA treatment, including GSTs. The transcriptional repression of GSTs is likely playing a key role in OTA toxicity via attenuation of glutathione conjugation/detoxification. The sequential molecular events may explain the mechanism of toxicity associated with OTA. Additionally, OTA transport studies using kidney MPS in the presence and absence of probenecid (1 mM) suggested a role for organic anionic membrane transporter(s) in the kidney specific disposition of OTA. Our findings provide a clearer understanding of the mechanism of OTA-induced kidney injury, which may support changes in risk assessment, regulatory agency policies on allowable exposure levels, and determination of the role of genetic factors in populations at risk for OTA nephrotoxicity.


































 京公网安备 11010802027423号
京公网安备 11010802027423号