Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-09-06 , DOI: 10.1016/j.scitotenv.2020.142155 Zeng-Hui Diao , Wei Chu
|
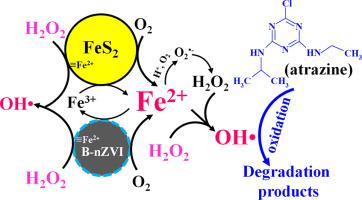
In this study, bentonite-supported nZVI (B-nZVI) was used as a catalyst to activate H2O2 for atrazine (ATZ) degradation in the presence of FeS2. Results indicated that ATZ degradation by B-nZVI/H2O2 process was significantly enhanced when FeS2 was introduced, and nearly 98% of ATZ was degraded by B-nZVI/FeS2/H2O2 process within 60 min under the optimum conditions. ATZ degradation of B-nZVI/FeS2/H2O2 process was much higher than the sum of B-nZVI and FeS2/H2O2 processes. The presence of HCO3−, PO43− and F− exhibited significant negative effects on the ATZ degradation, whereas both Cu2+ and Ni2+ exhibited positive effects on that. Both citric acid (CA) and ethylenediaminetetraacetic acid (EDTA) with lower concentration enhanced ATZ degradation rate, but significant suppression effects on that with higher concentration. The degradation of ATZ and 2,4-Dichlorophenol (2,4-DCP) could be simultaneously achieved in B-nZVI/FeS2/H2O2 process under certain conditions. High soluble Fe2+ induced an excellent decomposition of H2O2 by B-nZVI and FeS2. OH was dominant radical, and contributed to nearly 86% of the overall ATZ removal. A total of five intermediate products of ATZ were identified, and ATZ degradation was achieved via de-alkylation and hydroxylation processes. An enhanced reaction mechanism for ATZ degradation by B-nZVI/FeS2/H2O2 process was proposed, and B-nZVI/FeS2/H2O2 process exhibited an excellect catalytic performance within four successive runs.
was dominant radical, and contributed to nearly 86% of the overall ATZ removal. A total of five intermediate products of ATZ were identified, and ATZ degradation was achieved via de-alkylation and hydroxylation processes. An enhanced reaction mechanism for ATZ degradation by B-nZVI/FeS2/H2O2 process was proposed, and B-nZVI/FeS2/H2O2 process exhibited an excellect catalytic performance within four successive runs.
中文翻译:

膨润土负载的nZVI和过氧化氢在水中用FeS 2辅助降解at去津:性能和机理
在这项研究中,膨润土负载的nZVI(B-nZVI)被用作催化剂,在FeS 2存在下活化H 2 O 2来降解at去津(ATZ)。结果表明,引入FeS 2时,B-nZVI / H 2 O 2降解ATZ的能力明显增强,在60℃下60min内B-nZVI / FeS 2 / H 2 O 2降解近98%的ATZ。最佳条件。B-nZVI / FeS 2 / H 2 O 2工艺的ATZ降解远高于B-nZVI和FeS 2 / H 2 O 2的总和流程。HCO存在3 -,PO 4 3-和F -表现出对ATZ降解显著的负面影响,而这两个铜2+和Ni 2+显示出对积极的影响。较低浓度的柠檬酸(CA)和乙二胺四乙酸(EDTA)均可提高ATZ降解速率,但对较高浓度的ATZ降解速率具有明显的抑制作用。在某些条件下,B-nZVI / FeS 2 / H 2 O 2工艺可以同时实现ATZ和2,4-二氯苯酚(2,4-DCP)的降解。高可溶性Fe 2+引起H 2的出色分解O 2通过B-nZVI和FeS 2形成。OH 是主要的自由基,占全部ATZ去除量的近86%。总共鉴定出五种ATZ中间产物,并且通过脱烷基化和羟基化过程实现了ATZ降解。提出了通过B-nZVI / FeS 2 / H 2 O 2工艺降解ATZ的增强反应机理,并且B-nZVI / FeS 2 / H 2 O 2工艺在四个连续运行中表现出优异的催化性能。
是主要的自由基,占全部ATZ去除量的近86%。总共鉴定出五种ATZ中间产物,并且通过脱烷基化和羟基化过程实现了ATZ降解。提出了通过B-nZVI / FeS 2 / H 2 O 2工艺降解ATZ的增强反应机理,并且B-nZVI / FeS 2 / H 2 O 2工艺在四个连续运行中表现出优异的催化性能。













































 京公网安备 11010802027423号
京公网安备 11010802027423号