Molecular Therapy ( IF 12.1 ) Pub Date : 2020-09-05 , DOI: 10.1016/j.ymthe.2020.08.016 Yu-Yang Ng 1 , Johan C K Tay 1 , Zhendong Li 1 , Junjian Wang 2 , Jiangqing Zhu 2 , Shu Wang 1
|
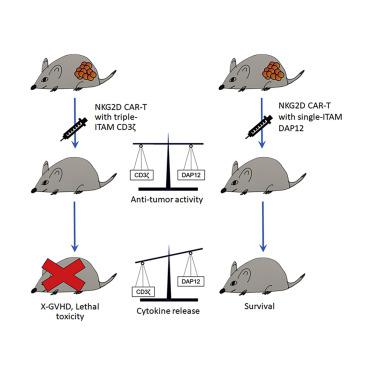
Cytokine-related toxicity associated with the use of highly active chimeric antigen receptor T cells (CAR-T cells) is a significant clinical problem. By fusing the natural killer group 2D (NKG2D) ectodomain to 4-1BB and the DAP12 cytoplasmic domain containing only one immunoreceptor tyrosine-based activation motif, we have developed a 2nd-generation (2nd-Gen) NKG2D CAR for stable expression in human T cells. When compared to T cells modified with NKG2D CAR containing the commonly used CD3ζ activation domain, T cells expressing the NKG2D-DAP12 CAR stimulated lower level release of interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin (IL)-2 during tumor cell lysis and their proliferative activity was lower upon repeated antigen stimulation, although no difference between the two CARs was observed in mediating in vitro tumor cell lysis. In tumor-bearing NSG mice, both types of CAR-T cells displayed similar anti-tumor activity, being able to completely eradicate established solid tumor xenografts. However, treatment with the NKG2D-CD3ζ CAR-T cells led to the death of most mice from xenogeneic graft versus host disease starting 30 days post-CAR-T cell injection, which was associated with a higher level of cytokine release, whereas all the mice treated with the NKG2D-DAP12 CAR-T cells survived well. Thus, the incorporation of the DAP12 activation domain in a CAR design may possibly provide a potential clinical advantage in mitigating the risk of cytokine release syndrome (CRS).
中文翻译:

表达具有 DAP12 信号域的 NKG2D CAR 的 T 细胞可刺激较低的细胞因子产生,同时有效根除肿瘤。
与使用高活性嵌合抗原受体 T 细胞(CAR-T 细胞)相关的细胞因子相关毒性是一个重要的临床问题。通过将自然杀伤组 2D (NKG2D) 胞外域与 4-1BB 和仅包含一个基于免疫受体酪氨酸的激活基序的 DAP12 胞质域融合,我们开发了第二代 (2nd-Gen) NKG2D CAR,用于在人类 T 中稳定表达细胞。与用含有常用 CD3ζ 激活结构域的 NKG2D CAR 修饰的 T 细胞相比,表达 NKG2D-DAP12 CAR 的 T 细胞刺激较低水平的干扰素 γ (IFN-γ)、肿瘤坏死因子 α (TNF-α) 和白细胞介素释放(IL)-2 在肿瘤细胞裂解过程中,其增殖活性在反复抗原刺激后较低,尽管在介导方面没有观察到两种 CAR 之间的差异体外肿瘤细胞裂解。在荷瘤 NSG 小鼠中,两种类型的 CAR-T 细胞表现出相似的抗肿瘤活性,能够完全根除已建立的实体瘤异种移植物。然而,用 NKG2D-CD3ζ CAR-T 细胞治疗导致大多数小鼠在 CAR-T 细胞注射后 30 天开始死于异种移植物抗宿主病,这与更高水平的细胞因子释放有关,而所有的用 NKG2D-DAP12 CAR-T 细胞治疗的小鼠存活良好。因此,在 CAR 设计中加入 DAP12 激活域可能会在降低细胞因子释放综合征 (CRS) 的风险方面提供潜在的临床优势。















































 京公网安备 11010802027423号
京公网安备 11010802027423号