当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A close competition between O–H⋯O and O–H⋯π hydrogen bonding: IR spectroscopy of anisole–methanol complex in helium nanodroplets
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-09-04 , DOI: 10.1039/d0cp02589e Tarun Kumar Roy 1, 2, 3, 4 , Devendra Mani 1, 2, 3, 4 , Gerhard Schwaab 1, 2, 3, 4 , Martina Havenith 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-09-04 , DOI: 10.1039/d0cp02589e Tarun Kumar Roy 1, 2, 3, 4 , Devendra Mani 1, 2, 3, 4 , Gerhard Schwaab 1, 2, 3, 4 , Martina Havenith 1, 2, 3, 4
Affiliation
|
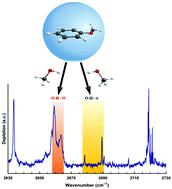
Anisole is a multifunctional molecule that can form intermolecular complexes via its aromatic π-electron system as well as its methoxy group. We have studied the complexation of anisole with methanol. This serves as a prototype system to explore the competition between O–H⋯O, O–H⋯π, C–H⋯O and C–H⋯π hydrogen bonding. The anisole⋯methanol molecular complexes were formed in superfluid helium droplets and were detected using high-resolution laser-infrared spectroscopy, in the frequency range between 2630 and 2730 cm−1 covering the O–D stretches of methanol-d4 (CD3OD). Several bands assigned to (anisole)m⋯(methanol)n complexes (where m = 1, and 2 and n = 1) were observed. The experimental results are complemented by the ab initio electronic structure calculations at the MP2/6-311++G(d,p) and B3LYP-D3/aug-cc-pVTZ levels of theory. Based on a comparison of the observed spectra with the ab initio theoretical spectra, we suggest that for the anisole⋯methanol complex, structures bound via O–H⋯O and O–H⋯π hydrogen bonding are almost equally preferred.
中文翻译:

O–H⋯O和O–H⋯π氢键之间的密切竞争:氦纳米液滴中苯甲醚-甲醇配合物的红外光谱
茴香醚是一种多功能分子,可以通过其芳香族π电子系统及其甲氧基形成分子间的配合物。我们已经研究了苯甲醚与甲醇的络合。这是探索O–H⋯O,O–H⋯π,C–H⋯O和C–H⋯π氢键之间竞争的原型系统。苯甲醚-甲醇分子复合物形成于超流体氦滴中,并通过高分辨率激光红外光谱法检测到,其频率范围为2630至2730 cm -1,覆盖了甲醇d 4的O–D延伸段(CD 3 OD )。分配给(苯甲醚)m ⋯(甲醇)n个配合物的几个谱带(其中m = 1、2和观察到n = 1)。实验结果得到MP2 / 6-311 ++ G(d,p)和B3LYP-D3 / aug-cc-pVTZ理论水平的从头算电子结构计算的补充。根据观察到的光谱与从头算理论光谱的比较,我们建议对于苯甲醚⋯甲醇配合物,通过O–H⋯O和O–H⋯π氢键键合的结构几乎同样优选。
更新日期:2020-10-16
中文翻译:

O–H⋯O和O–H⋯π氢键之间的密切竞争:氦纳米液滴中苯甲醚-甲醇配合物的红外光谱
茴香醚是一种多功能分子,可以通过其芳香族π电子系统及其甲氧基形成分子间的配合物。我们已经研究了苯甲醚与甲醇的络合。这是探索O–H⋯O,O–H⋯π,C–H⋯O和C–H⋯π氢键之间竞争的原型系统。苯甲醚-甲醇分子复合物形成于超流体氦滴中,并通过高分辨率激光红外光谱法检测到,其频率范围为2630至2730 cm -1,覆盖了甲醇d 4的O–D延伸段(CD 3 OD )。分配给(苯甲醚)m ⋯(甲醇)n个配合物的几个谱带(其中m = 1、2和观察到n = 1)。实验结果得到MP2 / 6-311 ++ G(d,p)和B3LYP-D3 / aug-cc-pVTZ理论水平的从头算电子结构计算的补充。根据观察到的光谱与从头算理论光谱的比较,我们建议对于苯甲醚⋯甲醇配合物,通过O–H⋯O和O–H⋯π氢键键合的结构几乎同样优选。

































 京公网安备 11010802027423号
京公网安备 11010802027423号