当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Small-Molecule Activity-Based Probe for Monitoring Ubiquitin C-terminal Hydrolase L1 (UCHL1) Activity in Live Cells and Zebrafish Embryos
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-04 , DOI: 10.1021/jacs.0c07726 Raymond Kooij 1 , Sijia Liu 1 , Aysegul Sapmaz 1 , Bo-Tao Xin 1 , George M C Janssen 2 , Peter A van Veelen 2 , Huib Ovaa 1 , Peter Ten Dijke 1 , Paul P Geurink 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-04 , DOI: 10.1021/jacs.0c07726 Raymond Kooij 1 , Sijia Liu 1 , Aysegul Sapmaz 1 , Bo-Tao Xin 1 , George M C Janssen 2 , Peter A van Veelen 2 , Huib Ovaa 1 , Peter Ten Dijke 1 , Paul P Geurink 1
Affiliation
|
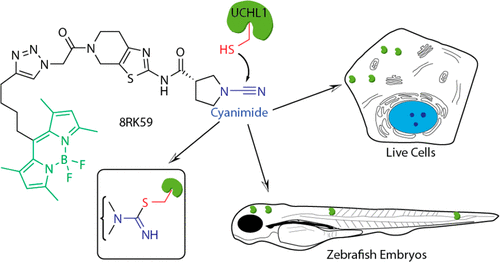
Many reagents have emerged to study the function of specific enzymes in vitro. On the other hand, target specific reagents are scarce or need improvement, allowing investigations of the function of individual enzymes in their native cellular context. Here we report the development of a target-selective fluorescent small-molecule activity-based DUB probe that is active in live cells and an in vivo animal model. The probe labels active ubiquitin carboxy-terminal hydrolase L1 (UCHL1), also known as neuron-specific protein PGP9.5 (PGP9.5) and Parkinson disease 5 (PARK5), a DUB active in neurons that constitutes 1 to 2% of the total brain protein. UCHL1 variants have been linked with neurodegenerative disorders Parkinson’s and Alzheimer’s diseases. In addition, high levels of UCHL1 also correlate often with cancer and especially metastasis. The function of UCHL1 activity or its role in cancer and neurodegenerative disease is poorly understood and few UCHL1-specific activity tools exist. We show that the reagents reported here are specific to UCHL1 over all other DUBs detectable by competitive activity-based protein profiling and by mass spectrometry. Our cell-penetrable probe, which contains a cyanimide reactive moiety, binds to the active-site cysteine residue of UCHL1 in an activity-dependent manner. Its use is demonstrated by the fluorescent labeling of active UCHL1 both in vitro and in live cells. We furthermore show that this probe can selectively and spatiotemporally report UCHL1 activity during the development of zebrafish embryos. Our results indicate that our probe has potential applications as a diagnostic tool for diseases with perturbed UCHL1 activity.
中文翻译:

一种基于小分子活性的探针,用于监测活细胞和斑马鱼胚胎中泛素 C 端水解酶 L1 (UCHL1) 的活性
已经出现了许多试剂来研究体外特定酶的功能。另一方面,目标特异性试剂很少或需要改进,允许研究单个酶在其天然细胞环境中的功能。在这里,我们报告了一种基于目标选择性荧光小分子活性的 DUB 探针的开发,该探针在活细胞和体内动物模型中具有活性。该探针标记活性泛素羧基末端水解酶 L1 (UCHL1),也称为神经元特异性蛋白 PGP9.5 (PGP9.5) 和帕金森病 5 (PARK5),这是一种在神经元中活跃的 DUB,占 1% 至 2%总脑蛋白。UCHL1 变体与神经退行性疾病帕金森病和阿尔茨海默病有关。此外,高水平的 UCHL1 也常常与癌症,尤其是转移相关。UCHL1 活性的功能或其在癌症和神经退行性疾病中的作用知之甚少,并且几乎没有 UCHL1 特定的活性工具存在。我们表明,与通过基于竞争活性的蛋白质分析和质谱法可检测到的所有其他 DUB 相比,此处报告的试剂对 UCHL1 具有特异性。我们的细胞可穿透探针含有氰酰亚胺反应部分,以活性依赖的方式与 UCHL1 的活性位点半胱氨酸残基结合。它的用途通过体外和活细胞中活性 UCHL1 的荧光标记来证明。我们进一步表明,该探针可以在斑马鱼胚胎发育过程中选择性地和时空报告 UCHL1 活性。我们的结果表明,我们的探针具有作为 UCHL1 活性受到干扰的疾病的诊断工具的潜在应用。
更新日期:2020-09-04
中文翻译:

一种基于小分子活性的探针,用于监测活细胞和斑马鱼胚胎中泛素 C 端水解酶 L1 (UCHL1) 的活性
已经出现了许多试剂来研究体外特定酶的功能。另一方面,目标特异性试剂很少或需要改进,允许研究单个酶在其天然细胞环境中的功能。在这里,我们报告了一种基于目标选择性荧光小分子活性的 DUB 探针的开发,该探针在活细胞和体内动物模型中具有活性。该探针标记活性泛素羧基末端水解酶 L1 (UCHL1),也称为神经元特异性蛋白 PGP9.5 (PGP9.5) 和帕金森病 5 (PARK5),这是一种在神经元中活跃的 DUB,占 1% 至 2%总脑蛋白。UCHL1 变体与神经退行性疾病帕金森病和阿尔茨海默病有关。此外,高水平的 UCHL1 也常常与癌症,尤其是转移相关。UCHL1 活性的功能或其在癌症和神经退行性疾病中的作用知之甚少,并且几乎没有 UCHL1 特定的活性工具存在。我们表明,与通过基于竞争活性的蛋白质分析和质谱法可检测到的所有其他 DUB 相比,此处报告的试剂对 UCHL1 具有特异性。我们的细胞可穿透探针含有氰酰亚胺反应部分,以活性依赖的方式与 UCHL1 的活性位点半胱氨酸残基结合。它的用途通过体外和活细胞中活性 UCHL1 的荧光标记来证明。我们进一步表明,该探针可以在斑马鱼胚胎发育过程中选择性地和时空报告 UCHL1 活性。我们的结果表明,我们的探针具有作为 UCHL1 活性受到干扰的疾病的诊断工具的潜在应用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号