当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arene Substitution Design for Controlled Conformational Changes of Dibenzocycloocta-1,5-dienes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-03 , DOI: 10.1021/jacs.0c06579 Wenxin Fu 1 , Todd M Alam 2 , Jiachen Li 3 , Jacqueline Bustamante 1 , Thanh Lien 4 , Ralph W Adams 5 , Simon J Teat 6 , Benjamin J Stokes 4 , Weitao Yang 3 , Yi Liu 7 , Jennifer Q Lu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-03 , DOI: 10.1021/jacs.0c06579 Wenxin Fu 1 , Todd M Alam 2 , Jiachen Li 3 , Jacqueline Bustamante 1 , Thanh Lien 4 , Ralph W Adams 5 , Simon J Teat 6 , Benjamin J Stokes 4 , Weitao Yang 3 , Yi Liu 7 , Jennifer Q Lu 1
Affiliation
|
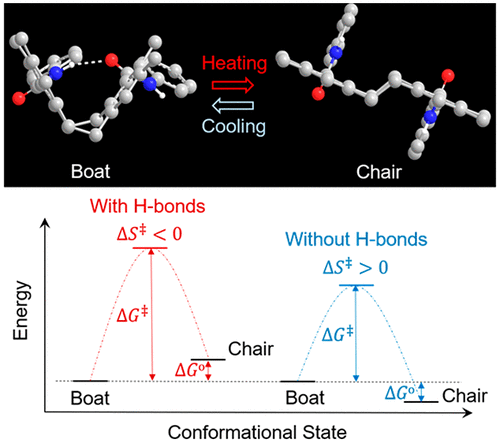
We report that the agile eight-membered cycloalkane can be stabilized by fusing two rigid benzene rings, substituted with proper functional groups. The conformational change of dibenzocycloocta-1,5-diene (DBCOD), a rigid-flexible-rigid organic moiety, from Boat to Chair conformation requires an activation energy of 42 kJ/mol that is substantially lower than that of existing submolecular shape-changing unit. Experimental data corroborated by theory calculations demonstrate that intramo-lecular hydrogen bonding can stabilize Boat whereas electron repulsive interaction from opposing ester substituents favors Chair. Intramolecular hydrogen bonding, formed by 1,10-diamide substitution stabilizes Boat, spiking the temperature at which Boat and Chair can readily interchange from -60 °C to 60 °C. Concomitantly this intramolecular attraction raises the energy barrier from 42 kJ/mol of unsubstituted DBCOD to 68 kJ/mol of diamide-substituted DBCOD. Remarkably, this value falls within the range of the activation energy of highly efficient enzyme catalyzed biological reactions. With shape changes once considered only possible with high-energy, our work reveals a potential pathway exemplified by a specific submolecu-lar structure to achieve low-energy driven shape changes for the first time. Together with intrinsic cycle stability and high energy output systems that would have incurred damage under high-energy stimuli, could particularly benefit from this new kind of low-energy driven shape-changing mechanism. This work has laid the basis to construct systems for low-energy driv-en stimuli-responsive applications, hitherto a challenge to overcome.
中文翻译:

芳烃取代设计控制二苯并环辛基 1,5-二烯的构象变化
我们报告说,灵活的八元环烷烃可以通过融合两个刚性苯环来稳定,并用适当的官能团取代。二苯并环辛基-1,5-二烯 (DBCOD) 的构象变化是一种刚性-柔性-刚性有机基团,从船形到椅形构象需要 42 kJ/mol 的活化能,这大大低于现有亚分子形状变化的活化能单元。理论计算证实的实验数据表明,分子内氢键可以稳定船,而来自相反酯取代基的电子排斥相互作用有利于主席。由 1,10-二酰胺取代形成的分子内氢键稳定船,使船和椅子可以轻松从 -60 °C 互换到 60 °C 的温度升高。同时,这种分子内吸引力将能量势垒从未取代的 DBCOD 的 42 kJ/mol 提高到二酰胺取代的 DBCOD 的 68 kJ/mol。值得注意的是,该值落在高效酶催化生物反应的活化能范围内。曾经认为只有高能量才有可能改变形状,我们的工作揭示了一种潜在途径,以特定的亚分子结构为例,首次实现低能量驱动的形状变化。再加上在高能刺激下会受到损害的内在循环稳定性和高能量输出系统,可以特别受益于这种新型低能量驱动的形状变化机制。这项工作为构建低能量驱动的刺激响应应用系统奠定了基础,
更新日期:2020-09-03
中文翻译:

芳烃取代设计控制二苯并环辛基 1,5-二烯的构象变化
我们报告说,灵活的八元环烷烃可以通过融合两个刚性苯环来稳定,并用适当的官能团取代。二苯并环辛基-1,5-二烯 (DBCOD) 的构象变化是一种刚性-柔性-刚性有机基团,从船形到椅形构象需要 42 kJ/mol 的活化能,这大大低于现有亚分子形状变化的活化能单元。理论计算证实的实验数据表明,分子内氢键可以稳定船,而来自相反酯取代基的电子排斥相互作用有利于主席。由 1,10-二酰胺取代形成的分子内氢键稳定船,使船和椅子可以轻松从 -60 °C 互换到 60 °C 的温度升高。同时,这种分子内吸引力将能量势垒从未取代的 DBCOD 的 42 kJ/mol 提高到二酰胺取代的 DBCOD 的 68 kJ/mol。值得注意的是,该值落在高效酶催化生物反应的活化能范围内。曾经认为只有高能量才有可能改变形状,我们的工作揭示了一种潜在途径,以特定的亚分子结构为例,首次实现低能量驱动的形状变化。再加上在高能刺激下会受到损害的内在循环稳定性和高能量输出系统,可以特别受益于这种新型低能量驱动的形状变化机制。这项工作为构建低能量驱动的刺激响应应用系统奠定了基础,


















































 京公网安备 11010802027423号
京公网安备 11010802027423号