当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of N‐Aryl Ethanolamines as N,O‐Ligand in Nickel(II)‐Catalyzed Carbon‐Carbon Bond Formation in Heck Coupling Reactions
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-09-03 , DOI: 10.1002/slct.202001578 Dattatraya S. Bhange 1, 2 , Rahul B. Sonawane 1 , Nishant K. Rasal 1 , Sangeeta V. Jagtap 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-09-03 , DOI: 10.1002/slct.202001578 Dattatraya S. Bhange 1, 2 , Rahul B. Sonawane 1 , Nishant K. Rasal 1 , Sangeeta V. Jagtap 1
Affiliation
|
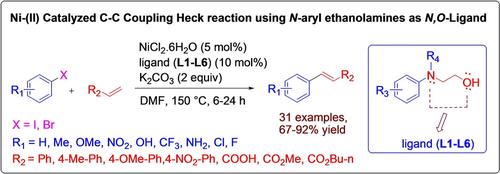
A simple and efficient protocol of Ni‐(II) catalyzed C−C bond formation in Heck coupling reactions of a variety of alkenes and aryl and hetero aryl halides, using various N‐aryl ethanolamines as bi‐dentate N,O‐ligand with moderate to excellent yields is described here. The application of different N‐aryl ethanolamines in presence of NiCl2.6H2O catalyst is tested for C−C bond formation in Heck reactions and found to be efficient in homogeneous medium. This simple and efficient protocol shows a broad substrate scope for substituted alkenes, aryl and hetero aryl halides and tolerates various functional groups and successfully demonstrated by 31 examples.
中文翻译:

N-芳基乙醇胺作为N,O-配体在Heck偶联反应中镍(II)催化的碳-碳键形成中的应用
使用各种N-芳基乙醇胺作为双齿N,O-配体,采用Ni-(II)的简单有效方案催化各种烯烃与芳基和杂芳基卤化物的Heck偶联反应中的C-C键形成此处描述了高产量。测试了在NiCl 2 .6H 2 O催化剂存在下使用不同的N-芳基乙醇胺在Heck反应中形成C-C键的效果,发现在均相介质中有效。这种简单而有效的方案显示了取代烯烃,芳基和杂芳基卤化物的广泛底物范围,并能耐受各种官能团,并通过31个实例成功证明。
更新日期:2020-09-03
中文翻译:

N-芳基乙醇胺作为N,O-配体在Heck偶联反应中镍(II)催化的碳-碳键形成中的应用
使用各种N-芳基乙醇胺作为双齿N,O-配体,采用Ni-(II)的简单有效方案催化各种烯烃与芳基和杂芳基卤化物的Heck偶联反应中的C-C键形成此处描述了高产量。测试了在NiCl 2 .6H 2 O催化剂存在下使用不同的N-芳基乙醇胺在Heck反应中形成C-C键的效果,发现在均相介质中有效。这种简单而有效的方案显示了取代烯烃,芳基和杂芳基卤化物的广泛底物范围,并能耐受各种官能团,并通过31个实例成功证明。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号