当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CeO2(111) Surface with Oxygen Vacancy for Radical Scavenging: A Density Functional Theory Approach
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-09-01 , DOI: 10.1021/acs.jpcc.0c05717 Robin Lawler 1, 2 , Jinwon Cho 1 , Hyung Chul Ham 3 , Hyunchul Ju 4 , Seung Woo Lee 5 , Jin Young Kim 6 , Ji Il Choi 1 , Seung Soon Jang 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-09-01 , DOI: 10.1021/acs.jpcc.0c05717 Robin Lawler 1, 2 , Jinwon Cho 1 , Hyung Chul Ham 3 , Hyunchul Ju 4 , Seung Woo Lee 5 , Jin Young Kim 6 , Ji Il Choi 1 , Seung Soon Jang 1
Affiliation
|
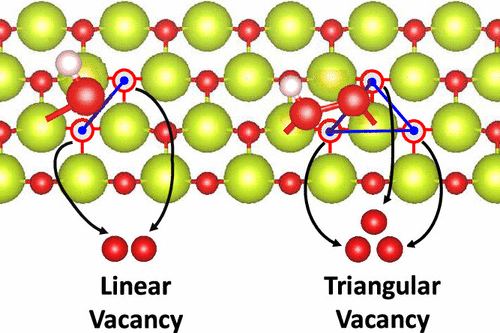
CeO2 has been established as an effective scavenger for destructive oxygen radicals in fuel cell membranes. The effect of ceria (CeO2) surface defect on •OH and •OOH radical scavenging efficacy is investigated using density functional theory (DFT). Our calculations suggest that both •OH and •OOH can be bound to oxygen vacancies on the CeO2(111) surface. Intriguingly, binding to the triangular defect (•OH binding energy = −4.54 eV; •OOH binding energy = −3.20 eV) is more favorable than binding to the linear defect (•OH binding energy = −4.12 eV; •OOH binding energy = −2.80 eV). This is likely due to Coulombic repulsive interaction from the additional oxygen atom adjacent to the linear defect. This is also potentially due to the greater localization of electrons to defect-adjacent cerium atoms in the triangular defect, as demonstrated via a Mulliken population analysis. Confirming this observation, it is shown that the density of states (DOS) for the Ce 4f band undergoes a significant alteration near the Fermi level due to this electron localization. As such, it is demonstrated that the triangular defect possesses a superior radical scavenging capability compared to the linear defect. Based on the results of this study, we suggest that future experimental studies aim to synthesize ceria nanoparticles with a greater percentage of triangular defects to enhance the particle radical scavenging capability.
中文翻译:

具有氧空位的CeO 2(111)表面用于自由基清除:密度泛函理论方法
已经建立了CeO 2作为燃料电池膜中破坏性氧自由基的有效清除剂。使用密度泛函理论(DFT)研究了二氧化铈(CeO 2)表面缺陷对• OH和• OOH自由基清除效率的影响。我们的计算表明,• OH和• OOH均可与CeO 2(111)表面的氧空位结合。有趣的是,与三角形缺陷的结合(• OH结合能= −4.54 eV;• OOH结合能= −3.20 eV)比与线性缺陷的结合(• OH结合能= −4.12 eV; •OOH结合能= -2.80eV。这可能是由于邻近线性缺陷的额外氧原子产生库仑排斥相互作用。这也可能是由于电子在三角形缺陷中更多地定位在与缺陷相邻的铈原子上,如Mulliken种群分析所证明的。证实了这一观察结果,表明由于该电子定位,Ce 4f能带的态密度(DOS)在费米能级附近发生了显着变化。这样,证明了与线性缺陷相比,三角形缺陷具有优异的自由基清除能力。根据这项研究的结果,
更新日期:2020-09-24
中文翻译:

具有氧空位的CeO 2(111)表面用于自由基清除:密度泛函理论方法
已经建立了CeO 2作为燃料电池膜中破坏性氧自由基的有效清除剂。使用密度泛函理论(DFT)研究了二氧化铈(CeO 2)表面缺陷对• OH和• OOH自由基清除效率的影响。我们的计算表明,• OH和• OOH均可与CeO 2(111)表面的氧空位结合。有趣的是,与三角形缺陷的结合(• OH结合能= −4.54 eV;• OOH结合能= −3.20 eV)比与线性缺陷的结合(• OH结合能= −4.12 eV; •OOH结合能= -2.80eV。这可能是由于邻近线性缺陷的额外氧原子产生库仑排斥相互作用。这也可能是由于电子在三角形缺陷中更多地定位在与缺陷相邻的铈原子上,如Mulliken种群分析所证明的。证实了这一观察结果,表明由于该电子定位,Ce 4f能带的态密度(DOS)在费米能级附近发生了显着变化。这样,证明了与线性缺陷相比,三角形缺陷具有优异的自由基清除能力。根据这项研究的结果,













































 京公网安备 11010802027423号
京公网安备 11010802027423号