当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Practical Synthetic Method for Functionalized 1-Methyl-3/5-(trifluoromethyl)-1H-pyrazoles
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.oprd.0c00300 Maxim A. Tairov 1 , Vitalina Levchenko 1 , Ivan A. Stadniy 1 , Yurii V. Dmytriv 1, 2 , Serhii O. Dehtiarov 1 , Mykola O. Kibalnyi 1 , Anton V. Melnyk 1 , Stanislav Y. Veselovych 1 , Yurii V. Borodulin 1 , Sergey V. Kolotilov 3 , Sergey V. Ryabukhin 1, 4 , Dmitriy M. Volochnyuk 1, 4, 5
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-09-02 , DOI: 10.1021/acs.oprd.0c00300 Maxim A. Tairov 1 , Vitalina Levchenko 1 , Ivan A. Stadniy 1 , Yurii V. Dmytriv 1, 2 , Serhii O. Dehtiarov 1 , Mykola O. Kibalnyi 1 , Anton V. Melnyk 1 , Stanislav Y. Veselovych 1 , Yurii V. Borodulin 1 , Sergey V. Kolotilov 3 , Sergey V. Ryabukhin 1, 4 , Dmitriy M. Volochnyuk 1, 4, 5
Affiliation
|
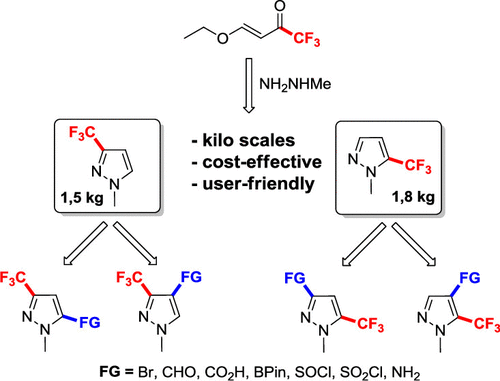
A new, high-yielding, and practical method for synthesis of 1-methyl-3-(trifluoromethyl)-1H-pyrazole and 1-methyl-5-(trifluoromethyl)-1H-pyrazole, key intermediates for important building blocks relevant to medicinal and agrochemistry, is developed. A one-step procedure for synthesis of the regioisomeric mixture of target pyrazoles was proposed starting from 4-ethoxy-1,1,1-trifluoro-3-buten-2-one. The procedure for separation of this mixture was elaborated on the basis of the boiling point vs pressure diagram analysis. The efficient synthetic strategies for regioisomeric building blocks bearing CF3 groups at the 3rd and 5th positions were demonstrated. A set of 1-methyl-3-(trifluoromethyl)-1H-pyrazoles containing such a functional group as aldehyde, acid, boron pinacolate, lithium sulfinate, and sulfonyl chloride was synthesized based on lithiation of 1-methyl-3-(trifluoromethyl)-1H-pyrazole in a flow reactor. Bromination of both 1-methyl-3-(trifluoromethyl)-1H-pyrazole and 1-methyl-5-(trifluoromethyl)-1H-pyrazole by NBS in mild conditions was performed. The introduction of the functional group at the 4th position of 1-methyl-5-(trifluoromethyl)-1H-pyrazole was illustrated by the optimized synthesis of the corresponding aldehyde and acid based on the Br–Li exchange in an appropriate bromide. Alternatively, the introduction of the functional group (acid and boron pinacolate) at the 5th position of 1-methyl-5-(trifluoromethyl)-1H-pyrazole was performed based on the direct ortho-metalation (DoM) reaction of 4-bromo-1-methyl-5-(trifluoromethyl)pyrazole followed by catalytic reductive debromination.
中文翻译:

官能化的1-甲基-3 / 5-(三氟甲基)-1 H-吡唑的实用合成方法
一种新的,高产且实用的合成1-甲基-3-(三氟甲基)-1H-吡唑和1-甲基-5-(三氟甲基)-1H-吡唑的重要实用方法到药物和农业化学的发展。从4-乙氧基-1,1,1-三氟-3-丁烯-2-一开始,提出了一步合成目标吡唑区域异构混合物的步骤。此混合物的分离步骤,阐述了沸点的基础上VS压力图分析。证明了在第3和第5位带有CF 3基团的区域异构结构单元的有效合成策略。一组1-甲基-3-(三氟甲基)-1 H基于1-甲基-3-(三氟甲基)-1 H-吡唑的锂化反应,合成了含有醛,酸,松果酸硼酸,亚磺酸锂和磺酰氯等官能团的β-吡唑。NBS在温和的条件下对1-甲基-3-(三氟甲基)-1 H-吡唑和1-甲基-5-(三氟甲基)-1 H-吡唑进行溴化。在1-甲基-5-(三氟甲基)-1 H的4位上引入官能团通过在适当的溴化物中进行Br-Li交换,优化了相应的醛和酸的合成,说明了吡唑。或者,基于4-溴的直接原位金属化(DoM)反应,在1-甲基-5-(三氟甲基)-1 H-吡唑的第5位上引入官能团(酸和松果酸硼)-1-甲基-5-(三氟甲基)吡唑,然后进行催化还原脱溴。
更新日期:2020-09-02
中文翻译:

官能化的1-甲基-3 / 5-(三氟甲基)-1 H-吡唑的实用合成方法
一种新的,高产且实用的合成1-甲基-3-(三氟甲基)-1H-吡唑和1-甲基-5-(三氟甲基)-1H-吡唑的重要实用方法到药物和农业化学的发展。从4-乙氧基-1,1,1-三氟-3-丁烯-2-一开始,提出了一步合成目标吡唑区域异构混合物的步骤。此混合物的分离步骤,阐述了沸点的基础上VS压力图分析。证明了在第3和第5位带有CF 3基团的区域异构结构单元的有效合成策略。一组1-甲基-3-(三氟甲基)-1 H基于1-甲基-3-(三氟甲基)-1 H-吡唑的锂化反应,合成了含有醛,酸,松果酸硼酸,亚磺酸锂和磺酰氯等官能团的β-吡唑。NBS在温和的条件下对1-甲基-3-(三氟甲基)-1 H-吡唑和1-甲基-5-(三氟甲基)-1 H-吡唑进行溴化。在1-甲基-5-(三氟甲基)-1 H的4位上引入官能团通过在适当的溴化物中进行Br-Li交换,优化了相应的醛和酸的合成,说明了吡唑。或者,基于4-溴的直接原位金属化(DoM)反应,在1-甲基-5-(三氟甲基)-1 H-吡唑的第5位上引入官能团(酸和松果酸硼)-1-甲基-5-(三氟甲基)吡唑,然后进行催化还原脱溴。






























 京公网安备 11010802027423号
京公网安备 11010802027423号