Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
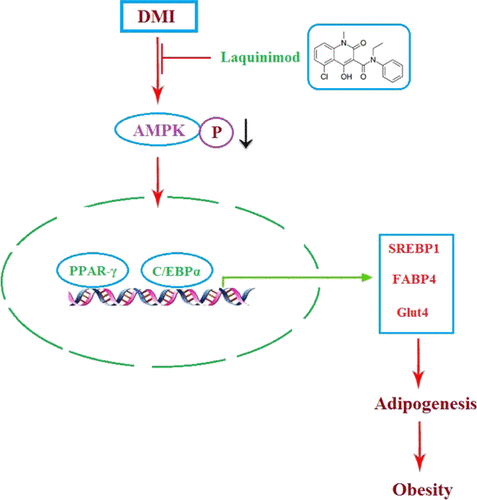
Laquinimod Prevents Adipogenesis and Obesity by Down-Regulating PPAR-γ and C/EBPα through Activating AMPK.
ACS Omega ( IF 3.7 ) Pub Date : 2020-09-01 , DOI: 10.1021/acsomega.0c02525 Guang Wang 1 , Bing Wu 2 , Lening Zhang 3 , Yang Cui 4 , Bo Zhang 5 , Heyuan Wang 6
ACS Omega ( IF 3.7 ) Pub Date : 2020-09-01 , DOI: 10.1021/acsomega.0c02525 Guang Wang 1 , Bing Wu 2 , Lening Zhang 3 , Yang Cui 4 , Bo Zhang 5 , Heyuan Wang 6
Affiliation
|
Background and Purpose: obesity is defined as excessive accumulation of adipose tissues and is becoming one of the main global severe public health issues. The present study aims to investigate the anti-adipogenesis of laquinimod and the underlying mechanism. Methods: a differentiation cocktail was used to differentiate 3T3-L1 cells, and mice were fed with high fat food to establish the obesity animal model. Oil red O staining, glycerol production assay, and the release of triglyceride were used to evaluate the differentiation degree of 3T3-L1 cells. The expression level of sterol regulatory element binding transcription factor 1 (Srebp1), fatty acid binding protein-4 (FABP4), glucose transporter 4 (GLUT4), peroxisome proliferator-activated receptor-γ (PPAR-γ), CCAAT enhancer-binding proteins (C/EBPα), and phosphorylation of adenosine 5′-monophosphate (AMP)-activated protein kinase α (p-AMPKα) was determined by quantitative real time PCRqRT-PCR and western blot analysis. The pathological state of adipose tissues was evaluated by hematoxylin–eosin staining. Results: the amount and UV absorption of oil red O, glycerol production, release of triglyceride, and the expression of SREBP1, FABP4, and Glut4 in differentiated 3T3-L1 cells were decreased by the administration of laquinimod. PPAR-γ and C/EBPα were down-regulated, and p-AMPKα was up-regulated by laquinimod. The down-regulated PPAR-γ and C/EBPα, as well as the inhibited lipid accumulation functioned by laquinimod, were reversed by the coincubation with the AMPK inhibitor compound C. Decreased body weight, visceral adipocyte tissue weight, and size of adipocytes were observed in in vivo obesity mice after administration with laquinimod. Conclusion: laquinimod might prevent adipogenesis by down-regulating PPAR-γ and C/EBPα through activating AMPK.
中文翻译:

拉喹莫德可通过激活AMPK来下调PPAR-γ和C /EBPα来预防脂肪形成和肥胖。
背景与目的:肥胖被定义为脂肪组织的过度积累,并且正在成为全球主要的严重公共卫生问题之一。本研究旨在研究拉喹莫德的抗脂肪形成及其潜在机制。方法:采用分化混合物分化3T3-L1细胞,给小鼠喂高脂食物建立肥胖动物模型。用油红O染色,甘油产生分析和甘油三酸酯的释放来评估3T3-L1细胞的分化程度。固醇调节元件结合转录因子1(Srebp1),脂肪酸结合蛋白4(FABP4),葡萄糖转运蛋白4(GLUT4),过氧化物酶体增殖物激活受体-γ(PPAR-γ),CCAAT增强剂结合蛋白的表达水平(C /EBPα),通过实时荧光定量PCRqRT-PCR和western blot分析确定腺苷5'-单磷酸(AMP)激活的蛋白激酶α(p-AMPKα)的磷酸化。用苏木精-伊红染色评估脂肪组织的病理状态。结果:拉喹莫德可降低分化的3T3-L1细胞中油红O的量和紫外线吸收,甘油生成,甘油三酸酯的释放以及SREBP1,FABP4和Glut4的表达。拉喹莫德可下调PPAR-γ和C /EBPα,而上调p-AMPKα。与AMPK抑制剂化合物C共孵育可以逆转下调的PPAR-γ和C /EBPα以及受拉喹莫德抑制的脂质积聚。体重,内脏脂肪细胞组织重量和脂肪细胞大小减少在 用苏木精-伊红染色评价脂肪组织的病理状态。结果:拉喹莫德可降低分化的3T3-L1细胞中油红O的量和紫外线吸收,甘油生成,甘油三酸酯的释放以及SREBP1,FABP4和Glut4的表达。拉喹莫德可下调PPAR-γ和C /EBPα,而上调p-AMPKα。与AMPK抑制剂化合物C共孵育可以逆转下调的PPAR-γ和C /EBPα以及受拉喹莫德抑制的脂质积聚。体重,内脏脂肪细胞组织重量和脂肪细胞大小减少在 用苏木精-伊红染色评价脂肪组织的病理状态。结果:拉喹莫德可降低分化的3T3-L1细胞中油红O的量和紫外线吸收,甘油生成,甘油三酸酯的释放以及SREBP1,FABP4和Glut4的表达。拉喹莫德可下调PPAR-γ和C /EBPα,而上调p-AMPKα。与AMPK抑制剂化合物C共孵育可以逆转下调的PPAR-γ和C /EBPα以及受拉喹莫德抑制的脂质积聚。体重,内脏脂肪细胞组织重量和脂肪细胞大小减少在 拉喹莫德可降低分化的3T3-L1细胞中的Glut4和Glut4。拉喹莫德可下调PPAR-γ和C /EBPα,而上调p-AMPKα。与AMPK抑制剂化合物C共孵育可以逆转下调的PPAR-γ和C /EBPα以及受拉喹莫德抑制的脂质积聚。体重,内脏脂肪细胞组织重量和脂肪细胞大小减少在 拉喹莫德可降低分化的3T3-L1细胞中的Glut4和Glut4。拉喹莫德可下调PPAR-γ和C /EBPα,而上调p-AMPKα。与AMPK抑制剂化合物C共孵育可以逆转下调的PPAR-γ和C /EBPα以及受拉喹莫德抑制的脂质积聚。体重,内脏脂肪细胞组织重量和脂肪细胞大小减少在拉喹莫德给药后的体内肥胖小鼠。结论:拉喹莫德可能通过激活AMPK来下调PPAR-γ和C /EBPα从而阻止脂肪形成。
更新日期:2020-09-15
中文翻译:

拉喹莫德可通过激活AMPK来下调PPAR-γ和C /EBPα来预防脂肪形成和肥胖。
背景与目的:肥胖被定义为脂肪组织的过度积累,并且正在成为全球主要的严重公共卫生问题之一。本研究旨在研究拉喹莫德的抗脂肪形成及其潜在机制。方法:采用分化混合物分化3T3-L1细胞,给小鼠喂高脂食物建立肥胖动物模型。用油红O染色,甘油产生分析和甘油三酸酯的释放来评估3T3-L1细胞的分化程度。固醇调节元件结合转录因子1(Srebp1),脂肪酸结合蛋白4(FABP4),葡萄糖转运蛋白4(GLUT4),过氧化物酶体增殖物激活受体-γ(PPAR-γ),CCAAT增强剂结合蛋白的表达水平(C /EBPα),通过实时荧光定量PCRqRT-PCR和western blot分析确定腺苷5'-单磷酸(AMP)激活的蛋白激酶α(p-AMPKα)的磷酸化。用苏木精-伊红染色评估脂肪组织的病理状态。结果:拉喹莫德可降低分化的3T3-L1细胞中油红O的量和紫外线吸收,甘油生成,甘油三酸酯的释放以及SREBP1,FABP4和Glut4的表达。拉喹莫德可下调PPAR-γ和C /EBPα,而上调p-AMPKα。与AMPK抑制剂化合物C共孵育可以逆转下调的PPAR-γ和C /EBPα以及受拉喹莫德抑制的脂质积聚。体重,内脏脂肪细胞组织重量和脂肪细胞大小减少在 用苏木精-伊红染色评价脂肪组织的病理状态。结果:拉喹莫德可降低分化的3T3-L1细胞中油红O的量和紫外线吸收,甘油生成,甘油三酸酯的释放以及SREBP1,FABP4和Glut4的表达。拉喹莫德可下调PPAR-γ和C /EBPα,而上调p-AMPKα。与AMPK抑制剂化合物C共孵育可以逆转下调的PPAR-γ和C /EBPα以及受拉喹莫德抑制的脂质积聚。体重,内脏脂肪细胞组织重量和脂肪细胞大小减少在 用苏木精-伊红染色评价脂肪组织的病理状态。结果:拉喹莫德可降低分化的3T3-L1细胞中油红O的量和紫外线吸收,甘油生成,甘油三酸酯的释放以及SREBP1,FABP4和Glut4的表达。拉喹莫德可下调PPAR-γ和C /EBPα,而上调p-AMPKα。与AMPK抑制剂化合物C共孵育可以逆转下调的PPAR-γ和C /EBPα以及受拉喹莫德抑制的脂质积聚。体重,内脏脂肪细胞组织重量和脂肪细胞大小减少在 拉喹莫德可降低分化的3T3-L1细胞中的Glut4和Glut4。拉喹莫德可下调PPAR-γ和C /EBPα,而上调p-AMPKα。与AMPK抑制剂化合物C共孵育可以逆转下调的PPAR-γ和C /EBPα以及受拉喹莫德抑制的脂质积聚。体重,内脏脂肪细胞组织重量和脂肪细胞大小减少在 拉喹莫德可降低分化的3T3-L1细胞中的Glut4和Glut4。拉喹莫德可下调PPAR-γ和C /EBPα,而上调p-AMPKα。与AMPK抑制剂化合物C共孵育可以逆转下调的PPAR-γ和C /EBPα以及受拉喹莫德抑制的脂质积聚。体重,内脏脂肪细胞组织重量和脂肪细胞大小减少在拉喹莫德给药后的体内肥胖小鼠。结论:拉喹莫德可能通过激活AMPK来下调PPAR-γ和C /EBPα从而阻止脂肪形成。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号