当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Process Development of the Weiss–Cook Reaction for the Preparation of cis-1,5-Dimethylbicyclo[3.3.0]octane-3,7-dione in the Undergraduate Organic Laboratory
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jchemed.9b00653
Matthew Korman 1 , Eric Paz 1 , Tylor Franklin 1 , Nicholas R. Lewandowski 1 , Bethany Sullivan 1 , Andrea M. Imhoff 1 , Luke Fisher 2 , Katherine A. Bichler 1 , Scott G. Van Ornum 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jchemed.9b00653
Matthew Korman 1 , Eric Paz 1 , Tylor Franklin 1 , Nicholas R. Lewandowski 1 , Bethany Sullivan 1 , Andrea M. Imhoff 1 , Luke Fisher 2 , Katherine A. Bichler 1 , Scott G. Van Ornum 1
Affiliation
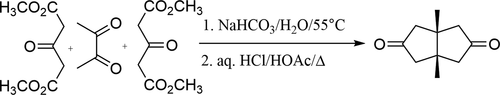
|
A four week laboratory experiment was designed on the basis of a modification of the Weiss–Cook reaction for the preparation of cis-1,5-dimethylbicyclo[3.3.0]octane-3,7-dione. In general, this very versatile organic reaction involves a two-step process reacting biacetyl with 2 mol of dimethyl-1,3-acetonedicarboxylate to form cis-bicycle([3.3.0]octane-3,7-dione). The reaction employs an aldol reaction followed by successive Michael reactions that are typically encountered near the end of a second-year undergraduate organic chemistry course. The students gained hands-on experience with 1H NMR and IR spectroscopy to analyze the intermediate and final product. Students wrote a formal laboratory report and discussed their results, including a proposal of the reaction mechanism and calculation of the overall yield. The experiment may be used in a traditional second semester organic chemistry laboratory or an advanced organic chemistry synthesis course on multigram or microscale levels.
中文翻译:

在大学有机实验室中,Weiss-Cook反应制备顺式-1,5-二甲基双环[3.3.0]辛烷-3,7-二酮的方法开发
在修改Weiss-Cook反应的基础上,设计了一个为期四周的实验室实验,以制备顺-1,5-二甲基双环[3.3.0]辛烷-3,7-二酮。通常,这种非常通用的有机反应涉及两步过程,使联乙酰与2摩尔1,3-丙酮二甲酸二甲酯反应形成顺式自行车([3.3.0]辛烷-3,7-二酮)。该反应采用醛醇缩合反应,随后是连续的迈克尔反应,通常在第二年本科有机化学课程即将结束时会遇到。学生们在实践中获得了11 H NMR和IR光谱分析中间产物和终产物。学生写了一份正式的实验室报告并讨论了他们的结果,包括反应机理的建议和总收率的计算。该实验可以在传统的第二学期有机化学实验室中使用,也可以在数克或微米级别的高级有机化学合成课程中使用。
更新日期:2020-10-13
中文翻译:

在大学有机实验室中,Weiss-Cook反应制备顺式-1,5-二甲基双环[3.3.0]辛烷-3,7-二酮的方法开发
在修改Weiss-Cook反应的基础上,设计了一个为期四周的实验室实验,以制备顺-1,5-二甲基双环[3.3.0]辛烷-3,7-二酮。通常,这种非常通用的有机反应涉及两步过程,使联乙酰与2摩尔1,3-丙酮二甲酸二甲酯反应形成顺式自行车([3.3.0]辛烷-3,7-二酮)。该反应采用醛醇缩合反应,随后是连续的迈克尔反应,通常在第二年本科有机化学课程即将结束时会遇到。学生们在实践中获得了11 H NMR和IR光谱分析中间产物和终产物。学生写了一份正式的实验室报告并讨论了他们的结果,包括反应机理的建议和总收率的计算。该实验可以在传统的第二学期有机化学实验室中使用,也可以在数克或微米级别的高级有机化学合成课程中使用。


































 京公网安备 11010802027423号
京公网安备 11010802027423号