当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Boundary and Salt Partitioning in Coacervate Complexes Formed between Poly(acrylic acid) and Poly(N,N-dimethylaminoethyl methacrylate) from Detailed Atomistic Simulations Combined with Free Energy Perturbation and Thermodynamic Integration Calculations
Macromolecules ( IF 5.1 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.macromol.0c00728 Dimitris G. Mintis 1 , Vlasis G. Mavrantzas 1, 2
Macromolecules ( IF 5.1 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.macromol.0c00728 Dimitris G. Mintis 1 , Vlasis G. Mavrantzas 1, 2
Affiliation
|
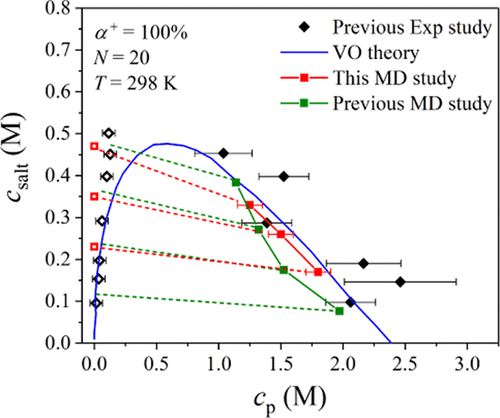
With the help of detailed atomistic simulations, we have determined the phase boundary of a complex coacervate system resulting from the complexation of two oppositely and fully charged weak polyelectrolytes, namely, poly(acrylic acid) (PAA) and poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA), characterized by degrees of polymerization N = 20 and 17, respectively, in an aqueous KCl solution. The binodal phase behavior was computed through an iterative procedure of balancing the total chemical potentials of water and salt in the supernatant and coacervate phases. Prior to any phase equilibria computations, several free energy calculation methods were evaluated in terms of their capability to provide accurate predictions of the excess chemical potential of water and salt at different temperatures. Methods based on free energy perturbation and thermodynamic integration were found to be the most reliable. Representative points on the salt–polymer binodal phase diagram were found to be in remarkably good agreement with the experimental data, as well as with a previous coarse-grained molecular dynamics (MD) study. We also studied the salt partitioning by directly simulating the two phases in contact one with the other at equilibrium, which showed a clear preference of salt ions for the polymer-lean phase; the predicted tie-lines exhibit negative slopes, denoting that the salt concentration in the supernatant phase is higher than in the coacervate phase. Additional results from the atomistic simulations regarding the pairs of atoms in the two oppositely charged polyelectrolytes that make the most important contributions to the complexation are also presented and discussed. Overall, our study suggests that fully detailed atomistic MD simulations can be successfully employed to predict with remarkable accuracy the phase behavior and salt partitioning of coacervate-based systems, thus offering significant insights into the complexation phenomenon and its underlying physics.
中文翻译:

结合自由能摄动和热力学积分计算的详细原子模拟,得出聚(丙烯酸)与聚(N,N-二甲基氨基乙基甲基丙烯酸甲酯)之间形成的凝聚层相的相界和盐分配
借助详细的原子模拟,我们确定了由两个相反且带正电荷的弱聚电解质(聚(丙烯酸)(PAA)和聚(N,N-二甲基氨基乙基))络合形成的复杂凝聚层系统的相界甲基丙烯酸酯(PDMAEMA),特征在于聚合度N在KCl水溶液中分别等于20和17。通过平衡上清液相和凝聚相中水和盐的总化学势的迭代程序,计算了回合相行为。在进行任何相平衡计算之前,先评估了几种自由能计算方法的能力,以提供对不同温度下水和盐的过量化学势的准确预测。发现基于自由能摄动和热力学积分的方法是最可靠的。发现盐-聚合物二元体相图上的代表点与实验数据以及先前的粗粒度分子动力学(MD)研究非常吻合。我们还通过直接模拟处于平衡状态的彼此接触的两相来研究盐分配,这表明盐离子对贫聚合物相具有明显的偏好。预测的联系线呈现负斜率,表明上清液相中的盐浓度高于凝聚层相中的盐浓度。还介绍和讨论了有关两种带相反电荷的聚电解质中成对原子的原子模拟的其他结果,这些成对作用对络合作用最重要。总体而言,我们的研究表明,可以使用详尽的原子MD模拟成功地以极高的精度预测基于凝聚层的系统的相行为和盐分配,
更新日期:2020-09-22
中文翻译:

结合自由能摄动和热力学积分计算的详细原子模拟,得出聚(丙烯酸)与聚(N,N-二甲基氨基乙基甲基丙烯酸甲酯)之间形成的凝聚层相的相界和盐分配
借助详细的原子模拟,我们确定了由两个相反且带正电荷的弱聚电解质(聚(丙烯酸)(PAA)和聚(N,N-二甲基氨基乙基))络合形成的复杂凝聚层系统的相界甲基丙烯酸酯(PDMAEMA),特征在于聚合度N在KCl水溶液中分别等于20和17。通过平衡上清液相和凝聚相中水和盐的总化学势的迭代程序,计算了回合相行为。在进行任何相平衡计算之前,先评估了几种自由能计算方法的能力,以提供对不同温度下水和盐的过量化学势的准确预测。发现基于自由能摄动和热力学积分的方法是最可靠的。发现盐-聚合物二元体相图上的代表点与实验数据以及先前的粗粒度分子动力学(MD)研究非常吻合。我们还通过直接模拟处于平衡状态的彼此接触的两相来研究盐分配,这表明盐离子对贫聚合物相具有明显的偏好。预测的联系线呈现负斜率,表明上清液相中的盐浓度高于凝聚层相中的盐浓度。还介绍和讨论了有关两种带相反电荷的聚电解质中成对原子的原子模拟的其他结果,这些成对作用对络合作用最重要。总体而言,我们的研究表明,可以使用详尽的原子MD模拟成功地以极高的精度预测基于凝聚层的系统的相行为和盐分配,































 京公网安备 11010802027423号
京公网安备 11010802027423号