当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NiFe-Layered Double Hydroxide Synchronously Activated by Heterojunctions and Vacancies for the Oxygen Evolution Reaction.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-08-31 , DOI: 10.1021/acsami.0c11847 Yang Luo 1, 2, 3 , Yinghong Wu 2, 3, 4 , Donghai Wu 5 , Chao Huang 1 , Dezhi Xiao 1 , Houyang Chen 6 , Shili Zheng 3 , Paul K Chu 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-08-31 , DOI: 10.1021/acsami.0c11847 Yang Luo 1, 2, 3 , Yinghong Wu 2, 3, 4 , Donghai Wu 5 , Chao Huang 1 , Dezhi Xiao 1 , Houyang Chen 6 , Shili Zheng 3 , Paul K Chu 1
Affiliation
|
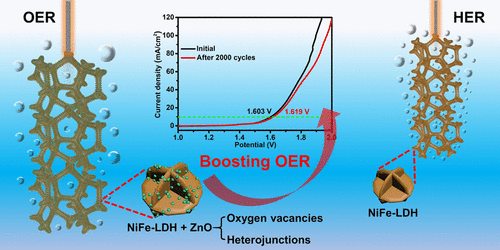
The development of earth-abundant transition-metal-based electrocatalysts with bifunctional properties (oxygen evolution reaction (OER) and hydrogen evolution reaction (HER)) is crucial to commercial hydrogen production. In this work, layered double hydroxide (LDH)-zinc oxide (ZnO) heterostructures and oxygen vacancies are constructed synchronously by plasma magnetron sputtering of NiFe-LDH. Using the optimal conditions, ZnO nanoparticles are uniformly distributed on the NiFe-LDH nanoflowers, which are prepared uniformly on the three-dimensional porous Ni foam. In the LDH-ZnO heterostructures and oxygen vacancies, electrons are depleted at the Ni cations on the NiFe-LDH surface and the active sites change from Fe cations to Ni cations during OER. Our theoretical assessment confirms the change of active sites after the deposition of ZnO and reveals the charge-transfer mechanism. Owing to the significant improvement in the OER dynamics, overall water splitting can be achieved at only 1.603 V in 1 M KOH when the Ni/LDH-ZnO and Ni/LDH are used as the anode and cathode, respectively. The work reveals a novel design of self-supported catalytic electrodes for efficient water splitting and also provides insights into the surface modification of catalytic materials.
中文翻译:

异质结和空位同步活化的NiFe层状双氢氧根,用于氧释放反应。
具有双功能性质(氧释放反应(OER)和氢释放反应(HER))的富含地球的过渡金属基电催化剂的开发对于工业制氢至关重要。在这项工作中,通过等离子磁控溅射NiFe-LDH来同步构造层状双氢氧化物(LDH)-氧化锌(ZnO)和氧空位。在最佳条件下,ZnO纳米颗粒均匀地分布在NiFe-LDH纳米花上,这些花是在三维多孔Ni泡沫上均匀制备的。在LDH-ZnO异质结构和氧空位中,电子在NiFe-LDH表面的Ni阳离子处耗尽,并且在OER过程中活性位从Fe阳离子变为Ni阳离子。我们的理论评估证实了ZnO沉积后活性位的变化,并揭示了电荷转移机制。由于OER动力学的显着改善,当分别将Ni / LDH-ZnO和Ni / LDH用作阳极和阴极时,在1 M KOH中仅1.603 V即可实现总水分解。这项工作揭示了一种自支撑催化电极的新颖设计,可进行有效的水分解,并为催化材料的表面改性提供了见识。
更新日期:2020-09-23
中文翻译:

异质结和空位同步活化的NiFe层状双氢氧根,用于氧释放反应。
具有双功能性质(氧释放反应(OER)和氢释放反应(HER))的富含地球的过渡金属基电催化剂的开发对于工业制氢至关重要。在这项工作中,通过等离子磁控溅射NiFe-LDH来同步构造层状双氢氧化物(LDH)-氧化锌(ZnO)和氧空位。在最佳条件下,ZnO纳米颗粒均匀地分布在NiFe-LDH纳米花上,这些花是在三维多孔Ni泡沫上均匀制备的。在LDH-ZnO异质结构和氧空位中,电子在NiFe-LDH表面的Ni阳离子处耗尽,并且在OER过程中活性位从Fe阳离子变为Ni阳离子。我们的理论评估证实了ZnO沉积后活性位的变化,并揭示了电荷转移机制。由于OER动力学的显着改善,当分别将Ni / LDH-ZnO和Ni / LDH用作阳极和阴极时,在1 M KOH中仅1.603 V即可实现总水分解。这项工作揭示了一种自支撑催化电极的新颖设计,可进行有效的水分解,并为催化材料的表面改性提供了见识。































 京公网安备 11010802027423号
京公网安备 11010802027423号