Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-08-30 , DOI: 10.1016/j.jhazmat.2020.123779
Fei Pan , Haodong Ji , Penghui Du , Taobo Huang , Chong Wang , Wen Liu
|
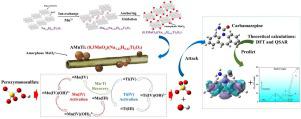
Developing efficient pharmaceuticals and personal care products (PPCPs) degradation technologies is of scientifical and practical importance to restrain their discharge into natural water environment. This study fabricated and applied a composite material of amorphous MnO2 nanoparticles in-situ anchored titanate nanotubes (AMnTi) to activate peroxymonosulfate (PMS) for efficient degradation and mineralization of carbamazepine (CBZ). The degradation pathway and toxicity evolution of CBZ during elimination were deeply evaluated through produced intermediates identification and theoretical calculations. AMnTi with a composition of (0.3MnO2)•(Na1.22H0.78Ti3O7) offered high activation efficiency of PMS, which exhibited 21- and 3-times degradation rate of CBZ compared with the pristine TNTs and MnO2, respectively. The high catalytic activity can be attributed to its unique structure, leading to a lattice shrinkage and small pores to confine the PMS molecule onto the interface. Therefore, efficient charge transfer and catalytic activation through MnO
Ti linkage occurred, and a Mn
Ti cycle mediating catalytic PMS activation was found. Both hydroxyl and sulfate radicals played key roles in CBZ degradation. Theoretical calculations, i.e., density functional theory (DFT) and computational toxicity calculations, combined with intermediates identification revealed that CBZ degradation pathway was hydroxyl addition and N
C cleavage. CBZ degradation in this system was also a toxicity-attenuation process.
中文翻译:

MnO 2纳米颗粒原位锚固钛酸酯纳米管对过硫酸单硫酸盐催化卡马西平降解的活化作用的见解:机理,生态毒性和DFT研究
开发有效的药品和个人护理产品(PPCP)降解技术对于限制其排放到天然水环境中具有科学和实践意义。这项研究制造并应用了无定形MnO 2纳米颗粒原位锚固的钛酸酯纳米管(AMnTi)的复合材料,以激活过氧单硫酸盐(PMS)来有效降解和矿化卡马西平(CBZ)。通过产生的中间体鉴定和理论计算,深入评估了消除过程中CBZ的降解途径和毒性演变。组成为(0.3MnO 2)•(Na 1.22 H 0.78 Ti 3 O 7的AMnTi)提供了较高的PMS活化效率,与原始TNT和MnO 2相比,CBZ的降解率分别为21倍和3倍。高催化活性可归因于其独特的结构,导致晶格收缩和小孔,从而将PMS分子限制在界面上。因此,发生了有效的电荷转移和通过Mn O
Ti键的催化活化,并且发现了一个
介导催化PMS活化的Mn Ti循环。羟基和硫酸根均在CBZ降解中起关键作用。理论计算,即密度泛函理论(DFT)和计算毒性计算,再结合中间体鉴定,表明CBZ降解途径为羟基加成和氮
C解理。该系统中的CBZ降解也是毒性减弱过程。




































 京公网安备 11010802027423号
京公网安备 11010802027423号