Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-08-31 , DOI: 10.1016/j.cej.2020.126795 Renyu Wang , Huijuan Liu , Kai Zhang , Gong Zhang , Huachun Lan , Jiuhui Qu
|
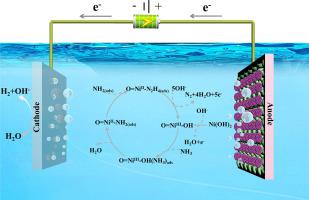
Converting ammonia in wastewater into harmless nitrogen is a green strategy, and electrochemical advanced oxidation processes (EAOP) based on electron transfer are an important means to realize this strategy. As a typical EAOP, ammonia oxidation catalyzed by high-valence transition metal anodes is one of the most effective and greenest conversion measures. Hence, in this study we constructed an electrocatalytic ammonia oxidation system using a nickel phosphide anode (Ni2P/NF). When the initial concentration of ammonia was 1000 mg l-1, and the current was 10 mA, the Faraday efficiency of Ni2P/NF in ammonia oxidation catalysis reached 52.8%. In addition, the Ni2P/NF anode could stabilize the electrolysis of ammonia for up to 24 hours. When the voltage was higher than 1.44 V vs. RHE, two peaks appeared at 479 cm-1 and 558 cm-1 in the in situ Raman spectrum and the corresponding current on the CV curve increased rapidly, which revealed that Ni oxyhydroxides formed on the reconstructed surface of Ni2P/NF were the real active sites for catalyzing the ammonia decomposition. The generated intermediates nitrate and nitrite were detected based on the in situ FTIR and spectrophotometric analysis. According to the experimental findings, we proposed a possible pathway for ammonia removal based on the participation of the Ni(II)/Ni(III) redox couple. This study enriched the in-depth understanding of ammonia oxidation and provided a very promising way to treat ammonia containing wastewater.
中文翻译:

Ni(II)/ Ni(III)氧化还原对赋予Ni泡沫支撑的Ni 2 P具有优异的直接氨氧化能力
将废水中的氨转化为无害氮是一种绿色策略,基于电子转移的电化学高级氧化工艺(EAOP)是实现该策略的重要手段。作为典型的EAOP,高价过渡金属阳极催化的氨氧化是最有效,最绿色的转化手段之一。因此,在这项研究中,我们使用磷化镍阳极(Ni 2 P / NF)构建了一个电催化氨氧化系统。当氨的初始浓度为1000 mg l -1,电流为10 mA时,Ni 2 P / NF的法拉第效率在氨氧化催化中达到52.8%。另外,Ni 2P / NF阳极可以稳定氨的电解长达24小时。当电压高于1.44 V vs. RHE时,在原位拉曼光谱中的479 cm -1和558 cm -1处出现两个峰,并且CV曲线上的相应电流迅速增加,这表明在Ni上形成了羟基氧化镍。 Ni 2 P / NF的重建表面是催化氨分解的真正活性中心。根据原位检测生成的中间体硝酸盐和亚硝酸盐FTIR和分光光度分析。根据实验结果,我们提出了一种基于Ni(II)/ Ni(III)氧化还原对参与脱氨的可能途径。这项研究丰富了对氨氧化的深入了解,为处理含氨废水提供了非常有希望的方法。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号