当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Study Investigating the Atmospheric Oxidation Mechanism and Kinetics of Dipropyl Thiosulfinate Initiated by OH Radicals and the Fate of Propanethiyl Radical.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-08-29 , DOI: 10.1021/acs.jpca.0c05200 Parandaman Arathala 1 , Rabi A Musah 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-08-29 , DOI: 10.1021/acs.jpca.0c05200 Parandaman Arathala 1 , Rabi A Musah 1
Affiliation
|
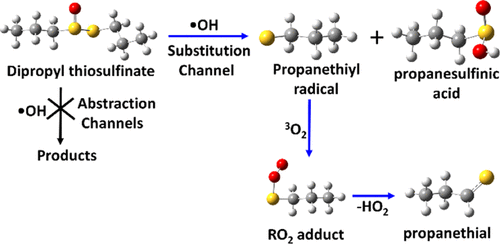
The OH radical-initiated atmospheric oxidation mechanism of dipropyl thiosulfinate (CH3CH2CH2–S(O)S–CH2CH2CH3, DPTS), a volatile released by Allium genus plants, has been investigated using ab initio/DFT electronic structure calculations. The DPTS + •OH reaction can proceed through (1) abstraction and (2) substitution pathways. The present calculations show that addition of •OH to the sulfur atom of the sulfinyl (−S(═O)) group, followed by simultaneous cleavage of the S–S single bond, leading to the formation of propanethiyl radical (PTR) and propanesulfinic acid, is the major pathway when compared to the other possible abstraction and substitution reactions. The barrier height for this reaction was computed to be −5.4 kcal mol–1 relative to that of the separated DPTS + •OH reactants. The rate coefficients for all the possible pathways for DPTS + •OH were explored by RRKM-ME calculations using the MESMER kinetic code in the atmospherically relevant temperatures T = 200–300 K and the pressure range of 0.1–10 atm. The calculated total rate coefficient for the DPTS + •OH reaction was found to be 1.7 × 10–10 cm3 molecule–1 s–1 at T = 300 K and P = 1 atm. The branching ratios and atmospheric lifetime of DPTS + •OH were also determined in the studied temperature range. In addition, electronic structure calculations on the multichannel reactions of PTR with atmospheric oxygen (3O2) were investigated using the same level of theory. The calculations showed that unimolecular elimination of hydroperoxyl radical (HO2) from the RO2 adduct through formation of propanethial is a major reaction under atmospherically relevant conditions. The overall results suggest that the atmospheric removal of DPTS is mainly due to reactions with •OH and 3O2, resulting in formation of propanesulfinic acid, propanethial, HO2, and sulfur dioxide (SO2) as the major products. The atmospheric lifetime of DPTS was estimated to be less than 2 h in the studied temperature range. Estimations of the global warming potential of DPTS and the products of its reaction with •OH reveal that while the contribution made by DPTS to global warming is negligible, the various products formed as a consequence of its interaction with OH radical may make substantial contributions to global warming, acid rain, and formation of secondary organic aerosols.
中文翻译:

计算研究调查了OH自由基引发的硫代二磺酸二丙酯的大气氧化机理和动力学以及丙乙氧基的去向。
二丙硫代亚磺酸酯的OH自由基引发大气氧化机制(CH 3 CH 2 CH 2 -S(O)S-CH 2 CH 2 CH 3,DPTS),易失性通过释放葱属植物,已经使用研究从头/ DFT电子结构计算。DPTS + • OH反应可以通过(1)抽象和(2)取代途径进行。目前的计算表明,•OH与亚磺酰基(-S(═O))基团的硫原子相连,然后同时裂解SS单键,导致形成丙硫基自由基(PTR)和丙烷亚磺酸,这是比较时的主要途径其他可能的抽象和替代反应。相对于分离的DPTS + • OH反应物,该反应的势垒高度经计算为-5.4 kcal mol –1。在大气相关温度T = 200–300 K和压力范围0.1–10 atm下,使用MESMER动力学代码通过RRKM-ME计算探索了DPTS + • OH的所有可能途径的速率系数。DPTS计算出的总速率系数+ •在T = 300 K和P = 1 atm时,发现OH反应为1.7×10 –10 cm 3分子–1 s –1。在所研究的温度范围内,还确定了DPTS + • OH的支化比和大气寿命。此外,使用相同水平的理论研究了PTR与大气氧(3 O 2)的多通道反应的电子结构计算。计算表明,从RO 2单分子消除了氢过氧自由基(HO 2)通过形成丙烷形成的加合物是在大气相关条件下的主要反应。总体结果表明,大气中DPTS的去除主要是由于与• OH和3 O 2的反应,导致形成了丙烷亚磺酸,丙烷,HO 2和二氧化硫(SO 2)作为主要产物。在研究的温度范围内,DPTS的大气寿命估计少于2小时。估计DPTS的全球变暖潜能及其与•的反应产物OH显示,尽管DPTS对全球变暖的贡献可以忽略不计,但由于其与OH自由基相互作用而形成的各种产品可能对全球变暖,酸雨和二次有机气溶胶的形成做出了重大贡献。
更新日期:2020-10-08
中文翻译:

计算研究调查了OH自由基引发的硫代二磺酸二丙酯的大气氧化机理和动力学以及丙乙氧基的去向。
二丙硫代亚磺酸酯的OH自由基引发大气氧化机制(CH 3 CH 2 CH 2 -S(O)S-CH 2 CH 2 CH 3,DPTS),易失性通过释放葱属植物,已经使用研究从头/ DFT电子结构计算。DPTS + • OH反应可以通过(1)抽象和(2)取代途径进行。目前的计算表明,•OH与亚磺酰基(-S(═O))基团的硫原子相连,然后同时裂解SS单键,导致形成丙硫基自由基(PTR)和丙烷亚磺酸,这是比较时的主要途径其他可能的抽象和替代反应。相对于分离的DPTS + • OH反应物,该反应的势垒高度经计算为-5.4 kcal mol –1。在大气相关温度T = 200–300 K和压力范围0.1–10 atm下,使用MESMER动力学代码通过RRKM-ME计算探索了DPTS + • OH的所有可能途径的速率系数。DPTS计算出的总速率系数+ •在T = 300 K和P = 1 atm时,发现OH反应为1.7×10 –10 cm 3分子–1 s –1。在所研究的温度范围内,还确定了DPTS + • OH的支化比和大气寿命。此外,使用相同水平的理论研究了PTR与大气氧(3 O 2)的多通道反应的电子结构计算。计算表明,从RO 2单分子消除了氢过氧自由基(HO 2)通过形成丙烷形成的加合物是在大气相关条件下的主要反应。总体结果表明,大气中DPTS的去除主要是由于与• OH和3 O 2的反应,导致形成了丙烷亚磺酸,丙烷,HO 2和二氧化硫(SO 2)作为主要产物。在研究的温度范围内,DPTS的大气寿命估计少于2小时。估计DPTS的全球变暖潜能及其与•的反应产物OH显示,尽管DPTS对全球变暖的贡献可以忽略不计,但由于其与OH自由基相互作用而形成的各种产品可能对全球变暖,酸雨和二次有机气溶胶的形成做出了重大贡献。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号