Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-08-30 , DOI: 10.1016/j.freeradbiomed.2020.08.018 Luke Carroll 1 , Shuwen Jiang 1 , Johanna Irnstorfer 1 , Sergi Beneyto 1 , Marta T Ignasiak 2 , Lars M Rasmussen 3 , Adelina Rogowska-Wrzesinska 4 , Michael J Davies 1
|
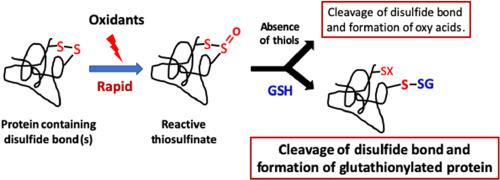
Disulfide bonds are a key determinant of protein structure and function, and highly conserved across proteomes. They are particularly abundant in extracellular proteins, including those with critical structural, ligand binding or receptor function. We demonstrate that oxidation of protein disulfides induces polymerization, and results in oxygen incorporation into the former disulfide via thiosulfinate generation. These intermediates, which have half-lives of several hours in vitro, undergo secondary reactions that cleave the disulfide bond, by irreversible hydrolysis to sulfinic and sulfonic acids, or reaction with thiols in a process that yields thiolated proteins (e.g. glutathionylated species in the case of reaction with glutathione). The adducts have been characterized by mass spectrometry (as ions corresponding to the addition of 306 and 712 Da for addition of one and two glutathione molecules, respectively) and immunoblotting. These modifications can be induced by multiple biologically-important oxidants, including HOCl, ONOOH, and H2O2, and on multiple proteins, demonstrating that this is a common disulfide modification pathway. Addition of glutathione to give glutathionylated proteins, can be reversed by reducing systems (e.g. tris(2-carboxyethyl)phosphine), but this does not repair the original disulfide bond. Exposure of human plasma to these modifying agents increases protein glutathionylation, demonstrating potential in vivo relevance. Overall these data provide evidence for a novel and facile route to glutathionylated proteins involving initial oxidation of a disulfide to a thiosulfinate followed by rapid reaction with GSH (‘oxidant-mediated thiol-disulfide exchange’). These data elucidate a novel pathway for protein glutathionylation that may have significant implications for redox biology and cell signaling.
中文翻译:

氧化剂诱导的蛋白质二硫键谷胱甘肽化。
二硫键是蛋白质结构和功能的关键决定因素,在整个蛋白质组中高度保守。它们在细胞外蛋白质中特别丰富,包括具有关键结构,配体结合或受体功能的蛋白质。我们证明蛋白质二硫化物的氧化诱导聚合,并导致氧通过硫代亚磺酸盐生成并入前二硫化物中。这些中间体在体外的半衰期为几个小时,通过不可逆地水解为亚磺酸和磺酸而经历裂解二硫键的次级反应,或在产生硫醇化蛋白质的过程中与硫醇反应(例如,在与谷胱甘肽反应的情况下为谷胱甘肽化物质)。加合物已经通过质谱法表征(作为分别对应于添加306和712Da分别添加一个和两个谷胱甘肽分子的离子)和免疫印迹。这些修饰可以由多种重要的生物氧化剂诱导,包括HOCl,ONOOH和H 2 O 2,并针对多种蛋白质,证明这是常见的二硫键修饰途径。可以通过还原系统(例如三(2-羧乙基)膦)逆转加入谷胱甘肽以产生谷胱甘肽化的蛋白质,但这不能修复原始的二硫键。人血浆暴露于这些修饰剂会增加蛋白质谷胱甘肽酰化,表明潜在的体内相关性。总体而言,这些数据提供了新颖而又简便的途径来制备谷胱甘肽化蛋白的证据,该方法涉及将二硫化物初始氧化为硫代亚磺酸盐,然后与GSH快速反应(“氧化剂介导的硫醇-二硫化物交换”)。这些数据阐明了蛋白质谷胱甘肽酰化的新途径,可能对氧化还原生物学和细胞信号传导具有重要意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号