Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-08-30 , DOI: 10.1016/j.cej.2020.126670 Olabode T. Ajenifujah , Amideddin Nouralishahi , Sarah Carl , Shawn C. Eady , Zhao Jiang , Levi T. Thompson
|
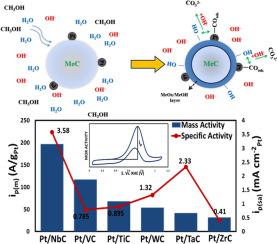
Some classes of electrocatalysts based on Pt supported early transition metal carbides (TMCs) have shown promise for methanol oxidation reaction (MOR). To bridge the material gap, we studied some of the promising and new electrocatalysts for MOR. We synthesized 1%wt Pt/TMCs (TMCs of group IV (Ti and Zr), group V (V, Nb and Ta) and group VI (W)) via wet impregnation method as low loading electrocatalysts for MOR in alkaline media. The synthesized materials were characterized by X-ray diffraction (XRD), inductively coupled plasma-optical emission spectroscopy (ICP-OES), N2 physisorption using Brunauer-Emmett-Teller (BET), transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The activity of the electrocatalysts were elucidated for MOR in alkaline media via combination of experimental and theoretical methods. Among all the investigated electrocatalysts, Pt/NbC was found to have the highest specific activity (3.58 mA cmPt−2) while Pt/ZrC had the least (0.410 mA cmPt−2). Tafel slope measurements for Pt/TMC electrocatalysts with the exception of Pt/TiC varied from region of low potential to region of high potential and were 121.2 ± 11 mV dec−1 and 234 ± 10 mV dec−1 respectively, indicating change in limiting steps from C-H scission to CO poisoning. Density functional theory (DFT) calculations of the binding energies of H and CO on the Pt/TMC surfaces were correlated to their specific activity, and volcano-type relationships were discovered, which indicated that neither too weak nor too strong bond of H and CO on the electrocatalyst surfaces were favorable for high activity during MOR. Finally, a feasible reaction mechanism for Pt/TMC electrocatalysts in MOR was proposed based on the experimental and theoretical results.
中文翻译:

早期过渡金属碳化物上载有铂:用于碱性电解液中甲醇电氧化反应的高效电催化剂
基于Pt负载的早期过渡金属碳化物(TMC)的某些类型的电催化剂显示出有望进行甲醇氧化反应(MOR)。为了弥合材料差距,我们研究了一些有前景的新型MOR电催化剂。我们通过湿法浸渍法合成了1%wt的Pt / TMCs(IV组(Ti和Zr),V组(V,Nb和Ta)和VI组(W)的TMCs)作为碱性介质中MOR的低负荷电催化剂。合成的材料通过X射线衍射(XRD),电感耦合等离子体发射光谱(ICP-OES),N 2进行表征使用Brunauer-Emmett-Teller(BET),透射电子显微镜(TEM)和X射线光电子能谱(XPS)进行物理吸附。通过实验和理论方法相结合的方式阐明了MOR在碱性介质中的电催化剂活性。在所有研究的电催化剂中,发现Pt / NbC具有最高的比活度(3.58 mA cm Pt -2),而Pt / ZrC具有最低的比活度(0.410 mA cm Pt -2)。除Pt / TiC以外,Pt / TMC电催化剂的塔菲尔斜率测量值从低电势区域到高电势区域变化,分别为121.2±11 mV dec -1和234±10 mV dec -1分别表明从CH分裂到CO中毒的限制步骤已发生变化。密度泛函理论(DFT)计算Pt / TMC表面上H和CO的结合能与它们的比活相关,并发现了火山类型的关系,这表明H和CO的键合既不弱也不强在电催化过程中,MOR期间的高活性是有利的。最后,基于实验和理论结果,提出了一种可行的反应机理,用于MOR中的Pt / TMC电催化剂。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号